
How to use Ebetrexat
Leaflet accompanying the packaging: patient information
Ebetrexat, 20 mg/ml, solution for injection in a pre-filled syringe
Methotrexate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Ebetrexat and what is it used for
- 2. Important information before using Ebetrexat
- 3. How to use Ebetrexat
- 4. Possible side effects
- 5. How to store Ebetrexat
- 6. Contents of the pack and other information
1. What is Ebetrexat and what is it used for
Ebetrexat is a medicine with the following properties:
- affects the growth of certain rapidly dividing cells (has anti-tumor effects);
- weakens unwanted immune reactions (has immunosuppressive effects);
- has anti-inflammatory effects.
Ebetrexat is used to treat patients with:
- active rheumatoid arthritis (RA) in adults;
- polyarticular forms (when 5 or more joints are affected) of severe active juvenile idiopathic arthritis (JIA), if treatment with non-steroidal anti-inflammatory drugs (NSAIDs) has been ineffective;
- severe, refractory, disabling psoriasis in patients with inadequate response to other types of treatment, such as phototherapy, photochemotherapy (PUVA) and retinoids, and with severe psoriatic arthritis (psoriatic arthritis) in adult patients.
2. Important information before using Ebetrexat
If you have any questions, consult your doctor or pharmacist before using Ebetrexat.
When not to use Ebetrexat
Warnings and precautions
Before starting treatment with Ebetrexat, discuss with your doctor if:
- you have diabetes and are being treated with insulin;
- you have a non-active, chronic infection (e.g. tuberculosis, hepatitis B or C, shingles);
- you have had liver or kidney disease in the past;
- you have lung function disorders;
- you have fluid accumulation in the abdominal cavity or in the space between the lungs and the chest (ascites, pleural effusion);
- you are dehydrated or have conditions leading to dehydration (vomiting, diarrhea, oral mucositis).
During methotrexate treatment, cases of acute pulmonary hemorrhage have been reported in patients with underlying rheumatologic disease. If you experience hemoptysis, i.e. coughing up bloody sputum, you should immediately consult your doctor.
The medicine should be used once a week.
Incorrect use of methotrexate can lead to severe, potentially life-threatening side effects.
It is very important to carefully read section 3 of this leaflet.
Methotrexate may increase skin sensitivity to sunlight. You should avoid intense sun exposure and not use a sunbed or sunlamp without consulting your doctor.
To protect your skin from intense sunlight, you should wear appropriate clothing or use a sunscreen with a high protection factor.
During methotrexate treatment, a recurrence of radiation-induced skin inflammation (radiation recall) or sunburn (so-called "recall reaction") may occur.
If you, your partner, or caregiver notice new or worsening neurological symptoms, including general muscle weakness, vision disturbances, changes in thinking, memory, and orientation leading to disorientation and personality changes, you should immediately consult your doctor, as these may be symptoms of a very rare, serious brain infection called progressive multifocal leukoencephalopathy (PML).
Children and adolescents
Dosing is based on the patient's body weight. It is not recommended to use the medicine in patients under 3 years of age due to insufficient experience in this age group.
The use of Ebetrexat in children should be carefully controlled by an experienced specialist to recognize potential side effects as early as possible.
Elderly patients
During methotrexate treatment, elderly patients should be carefully monitored to recognize potential side effects as early as possible. Due to age-related liver and kidney function disorders and decreased folate reserve in the body, methotrexate doses used in elderly patients should be relatively small.
Exposure to UV radiation during Ebetrexat therapy may exacerbate psoriatic skin lesions.
Recommended monitoring tests and precautions
Even with low doses of Ebetrexat, severe side effects may occur. To detect these effects early, your doctor will perform monitoring tests and laboratory tests.
Before starting treatment
Before starting treatment, blood tests will be performed to check if the blood cell count is sufficient. The blood will also be tested to check liver function and to determine if there is hepatitis. Additionally, serum albumin (a protein in the blood), liver inflammation (hepatitis infection), and kidney function will be checked. Your doctor may also decide to perform other liver tests, some of which may be imaging tests of the organ, and others may require taking a small tissue sample for further examination. Your doctor may also check if you have tuberculosis and order a chest X-ray or lung function test.
During treatment:
Your doctor may perform the following tests:
- examination of the mouth and throat for changes in the mucous membrane, such as inflammation or ulcers
- blood tests/morphology with blood cell count and measurement of methotrexate concentration in serum
- blood test to monitor liver function
- imaging tests to monitor liver condition
- taking a small liver sample for further examination
- blood test to monitor kidney function
- monitoring of the respiratory system and, if necessary, lung function test.
It is very important that you attend these scheduled tests.
If the results of any of these tests are abnormal, your doctor will modify the treatment accordingly.
Special precautions for using Ebetrexat
Methotrexate temporarily disrupts sperm and egg cell production; in most cases, this effect disappears. Methotrexate can cause miscarriage and severe birth defects. Female patients should avoid becoming pregnant during methotrexate treatment and for at least 6 months after treatment ends, using reliable contraception methods throughout this period (see also "Pregnancy, breastfeeding and fertility").
Male patients should avoid fathering a child during methotrexate treatment and for at least 3 months after treatment ends. See also "Pregnancy, breastfeeding and fertility".
Ebetrexat and other medicines
Tell your doctor or pharmacist about all medicines you are currently taking, have recently taken, or plan to take. If your doctor prescribes you other medicines, inform them about your use of Ebetrexat.
It is especially important to inform your doctor about the use of:
- other medicines used for rheumatoid arthritis or psoriasis, such as leflunomide, sulfasalazine (also used to treat ulcerative colitis), acetylsalicylic acid, phenylbutazone, or aminophenazone;
- alcohol (should be avoided);
- live vaccines;
- azathioprine (used to prevent transplant rejection);
- retinoids (used to treat psoriasis and other skin diseases);
- antiepileptic drugs (used to prevent seizures);
- anticancer drugs;
- barbiturates (sleeping pills given by injection);
- sedatives;
- oral contraceptives;
- probenecid (used to treat gout);
- antibiotics;
- penicillin - may decrease methotrexate excretion, potentially increasing side effects;
- metamizole (synonyms: novaminsulfone and dipyrone) (strong pain reliever and/or antipyretic);
- pyrimethamine (used to prevent and treat malaria);
- vitamin preparations containing folic acid;
- proton pump inhibitors (used to treat severe heartburn or ulcers);
- theophylline (used to treat asthma).
Ebetrexat with food, drink, and alcohol
During Ebetrexat treatment, you should avoid consuming alcohol and excessive amounts of coffee, caffeinated beverages, and black tea. During Ebetrexat treatment, you should drink plenty of fluids, as dehydration (reduced water content in the body) can increase the toxic effects of Ebetrexat.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
Do not use Ebetrexat if you are pregnant or trying to become pregnant. Methotrexate can cause birth defects, harm the unborn child, or cause miscarriage.
This is related to developmental abnormalities of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that female patients who are pregnant or planning to become pregnant do not take methotrexate. If you are of childbearing age, it is essential to confirm that you are not pregnant before starting treatment, taking appropriate measures, such as a pregnancy test.
Female patients should avoid becoming pregnant during methotrexate treatment and for at least 6 months after treatment ends, using reliable contraception methods throughout this period (see also "Warnings and precautions").
If you become pregnant during treatment or think you may be pregnant, consult your doctor as soon as possible. You should receive advice on the potential harmful effects of treatment on the child.
If you plan to become pregnant, consult your treating doctor, who may refer you to a specialist for advice before planned treatment begins.
Breastfeeding
Do not breastfeed during treatment, as methotrexate passes into breast milk. If your treating doctor considers methotrexate treatment essential at this time, you should stop breastfeeding.
Male fertility
Available evidence does not indicate an increased risk of birth defects or miscarriages after paternal exposure to methotrexate at a dose below 30 mg/week. However, this risk cannot be entirely excluded. Methotrexate may be genotoxic, meaning it can cause genetic mutations. Methotrexate may affect sperm and cause birth defects. Therefore, male patients should avoid fathering a child during methotrexate treatment and for at least 3 months after treatment ends.
Driving and using machines
During Ebetrexat treatment, side effects such as fatigue and dizziness may occur, which can impair your ability to drive and use machines. If you experience fatigue or dizziness, you should not drive or operate machines.
Ebetrexat contains sodium chloride and sodium hydroxide
This medicinal product contains less than 1 mmol (23 mg) of sodium per weekly dose, i.e., it is considered "sodium-free".
3. How to use Ebetrexat
Important warning regarding Ebetrexat dosing (methotrexate):
In the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and psoriasis, Ebetrexat should be used only once a week. Using more Ebetrexat (methotrexate) than prescribed can be fatal. It is very important to carefully read section 3 of this leaflet. If you have any questions, consult your doctor or pharmacist before taking the medicine.
Ebetrexat can only be prescribed by doctors familiar with the properties of this medicine and its mechanism of action.
This medicine should always be used exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist.
Ebetrexat is administered only once a week. You and your doctor should choose a suitable day of the week for the injection.
Incorrect use of Ebetrexat can lead to severe side effects, which can be fatal.
Typically, the recommended dose is:
Dosing in patients with rheumatoid arthritis
The recommended initial dose is 7.5 mg of methotrexate once a week. Ebetrexat is administered in a single subcutaneous, intramuscular, or intravenous injection (see "Method of administration and duration of treatment").
If Ebetrexat is not sufficiently effective and the patient tolerates it well, the doctor may increase the dose. The average weekly dose of methotrexate is 15-20 mg.
Generally, the weekly dose should not exceed 25 mg. After achieving the desired therapeutic effect, the dose should be gradually reduced to the smallest effective maintenance dose, if possible.
Dosing in children and adolescents under 16 years of age with polyarticular forms of juvenile idiopathic arthritis
The recommended dose is 10-15 mg/m² body surface area (BSA) per week. If the response to treatment is inadequate, the doctor may increase the weekly dose to 20 mg/m² BSA per week. More frequent monitoring tests are necessary in this case. Due to the limited amount of data on intravenous administration in children and adolescents, methotrexate should only be administered subcutaneously and intramuscularly.
The use of Ebetrexat is not recommended in children under 3 years of age due to insufficient experience in this age group.
Adults with severe psoriasis or psoriatic arthritis
A single test dose of 5-10 mg is recommended to assess potential toxic effects. This dose can be administered subcutaneously, intramuscularly, or intravenously.
If no changes in blood cell count occur after one week, treatment can be continued with a dose of approximately 7.5 mg. This dose can be gradually increased (by 5-7.5 mg per week, monitoring blood cell count) to achieve optimal treatment results. A weekly dose of 20 mg may be associated with significant increased toxicity. The dose should not exceed 30 mg per week.
After achieving the desired therapeutic effect, the dose should be reduced weekly to the smallest effective maintenance dose for the individual patient.
Patients with kidney function disorders
In patients with kidney function disorders, it may be necessary to reduce the dose of Ebetrexat.
Method of administration and duration of treatment
The duration of treatment is determined by your doctor. Ebetrexat is used in an injection once a week!It is recommended to choose one day of the week for the injection. Ebetrexat is administered subcutaneously, intramuscularly, or intravenously. Children and adolescents should not be given Ebetrexat intravenously.
Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis vulgaris, and psoriatic arthritis requires long-term use of Ebetrexat.
Rheumatoid arthritis
Improvement of symptoms is usually observed after 4-8 weeks of treatment. After discontinuation of Ebetrexat, symptoms may recur.
Severe forms of psoriasis vulgaris and psoriatic arthritis (psoriasis arthropatica)
Expected response to treatment usually occurs after 2-6 weeks.
Depending on the severity of symptoms and laboratory values, treatment should be continued or discontinued.
Initially, Ebetrexat may be administered by medical personnel. However, your doctor may decide that you are able to self-administer Ebetrexat injections. You will be properly trained in this regard. Never attempt to self-administer the injection without prior training.
Using a higher dose of Ebetrexat than recommended
Use the dose prescribed by your doctor. Never change it yourself.
If you suspect that you (or someone else) have used a higher dose of the medicine than you should, immediately contact your doctor or the emergency department of the nearest hospital. Only your doctor can decide on the type of treatment to be undertaken, depending on the severity of the overdose symptoms.
Methotrexate overdose can cause severe toxic reactions. Symptoms of overdose include: easy bruising or bleeding, unexplained weakness, mouth sores, nausea, vomiting, black or bloody stools, coughing up blood or vomiting coffee-ground-like material, and decreased urine output. See also section 4.
Patients reporting to the hospital or doctor should bring the medicine packaging with them.
The antidote used in case of poisoning is calcium folinate.
Missing a dose of Ebetrexat
Do not take a double dose to make up for a missed dose. Continue taking the recommended dose of the medicine. Consult your doctor for advice.
Stopping Ebetrexat treatment
Do not stop or discontinue Ebetrexat treatment without consulting your doctor.
If you suspect severe side effects, immediately seek medical advice.
If you have any further questions or concerns about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Ebetrexat can cause side effects, although not everybody gets them.
Immediately inform your doctor if you experience sudden wheezing, difficulty breathing, swelling of the eyelids, face, or lips, rash, or itching (especially if it spreads over the whole body) and fainting (these may be symptoms of severe allergic reactions or anaphylactic shock).
Severe side effects
Immediately consult your doctor if you experience the following side effects:
- respiratory problems (symptoms may include general malaise; dry, irritating cough; shortness of breath, dyspnea at rest, chest pain, or fever);
- hemoptysis, i.e., coughing up bloody sputum;
- severe skin peeling or blistering (also in the mouth, eyes, and genital area);
- unexplained bleeding (including bloody vomiting) or bruising;
- severe diarrhea;
- mouth ulcers;
- black or bloody stools;
- blood in urine or stool;
- small red spots on the skin;
- fever;
- jaundice (yellowing of the skin);
- pain or difficulty urinating;
- feeling thirsty and/or frequent urination;
- seizures (convulsions);
- loss of consciousness;
- blurred vision or vision disturbances.
The following side effects have also been reported:
Very common (may affect more than 1 in 10 people):
- loss of appetite, nausea, vomiting, abdominal pain
- inflammation and ulcers of the mouth and throat
- increased liver enzyme activity.
Common (may affect up to 1 in 10 people):
- decreased production of blood cells with decreased white blood cell, red blood cell, or platelet count (leukopenia, anemia, thrombocytopenia)
- headache
- feeling tired, drowsy
- tingling, prickling, or burning sensation of the skin, rash, redness of the skin, itching
- pneumonia
- diarrhea.
Uncommon (may affect up to 1 in 100 people):
- shingles
- lymphoma (which may resolve after discontinuation of the medicine)
- decreased blood cell and platelet count
- severe allergic reactions
- diabetes
- depression
- weakness of the muscles on one side of the body
- dizziness, disorientation
- seizures
- brain damage (leukoencephalopathy/encephalopathy)
- vasculitis
- lung damage, fluid accumulation in the space around the lungs
- ulcers and bleeding in the digestive tract
- pancreatitis
- liver function disorders
- decreased protein levels in the blood
- hives (without other symptoms), increased skin sensitivity to sunlight, brown discoloration of the skin
- severe toxic skin reactions, including blistering and peeling (Stevens-Johnson syndrome, Lyell syndrome)
- hair loss
- enlargement of rheumatoid nodules
- painful psoriatic lesions
- reactions resembling sunburn due to increased skin sensitivity to sunlight
- joint or muscle pain
- osteoporosis (decreased bone mass)
- inflammation and ulcers of the bladder (which may be accompanied by blood in the urine), painful urination
- birth defects
- inflammation and ulcers of the vagina
- burning or damage to tissue after intramuscular injection.
Rare (may affect up to 1 in 1,000 people):
- sepsis
- presence of very large red blood cells in the blood (megaloblastic anemia)
- mood swings
- transient perception disorders
- weakness of purposeful movements of all muscles
- difficulty speaking
- severe eye disorders
- low blood pressure
- blood clots
- sore throat
- respiratory arrest
- inflammation of the digestive tract, bloody stools
- inflammation of the gums
- acute hepatitis
- change in nail color, nail loss
- acne, presence of red or purple spots due to bleeding
- stress fractures
- electrolyte disturbances
- miscarriage
- abnormal sperm production
- menstrual disorders.
Very rare (may affect up to 1 in 10,000 people):
- herpes labialis
- hepatitis
- severe bone marrow failure
- immunodeficiency (hypogammaglobulinemia)
- pain
- muscle weakness
- taste disorders (metallic taste in the mouth)
- meningitis (inflammation of the membranes covering the brain), causing paralysis or vomiting
- redness of the eyes
- pericarditis (inflammation of the "bag" surrounding the heart), fluid in the pericardium
- pneumonitis, difficulty breathing, asthma
- bloody vomiting
- liver failure
- paronychia (infection of the nail bed), furuncles, presence of small blood vessels visible on the skin
- protein in the urine
- fetal death
- disorders of ovum maturation in women and sperm production in men
- decreased libido
- erectile dysfunction
- leukorrhea
- infertility
- mild skin reactions after subcutaneous administration of the medicine
- lymphoproliferative disorders (overproduction of white blood cells)
- feeling of numbness, tingling, or reduced reaction to stimuli.
Frequency not known (cannot be estimated from the available data):
- infections, which can be life-threatening in some cases
- lymph node swelling
- immune system disorders
- fever
- vasculitis due to allergic reaction
- peritonitis (inflammation of the membrane lining the abdominal cavity)
- slow wound healing
- pulmonary hemorrhage
- jawbone damage (due to overproduction of white blood cells)
- tissue damage at the injection site
- redness and peeling of the skin
- edema.
After intramuscular administration, side effects (burning sensation) or tissue damage (sterile abscess, fat tissue atrophy) may occur frequently at the injection site.
Subcutaneous administration of methotrexate is well tolerated. Only mild skin reactions have been observed, which decrease during continued treatment.
Methotrexate may decrease the white blood cell count and weaken the immune system, making it easier to develop infections. If you develop an infection with symptoms such as fever and significant deterioration of your general condition, or fever and symptoms of local infection (sore throat and/or pharyngitis), or urinary disorders, you should immediately consult your doctor. Your doctor will order blood tests to determine if your white blood cell count has decreased (if you have developed agranulocytosis). It is essential to inform your doctor about your use of Ebetrexat.
Methotrexate can cause severe (sometimes life-threatening) side effects. Therefore, your doctor will order tests to detect potential changes in your blood count (e.g., decreased white blood cell count, low platelet count, presence of lymphoma) and changes in your kidneys and liver.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Ebetrexat
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label of the pre-filled syringe and on the carton after EXP. The expiry date refers to the last day of the month.
Store the pre-filled syringe in the outer packaging to protect it from light.
Do not store above 25°C.
Use the medicine immediately after opening.
Do not use Ebetrexat if the solution is not clear or contains precipitated particles.
The medicine is for single use only. Any remaining solution should be disposed of.
Do not throw away any medicines or their packaging via wastewater or household waste. Ask your pharmacist how to dispose of medicines or packaging you no longer need. This will help protect the environment.
6. Contents of the pack and other information
What Ebetrexat contains
The active substance is methotrexate.
1 ml of solution for injection contains 20 mg of methotrexate (as 21.94 mg of methotrexate disodium)
1 pre-filled syringe with 0.375 ml of solution contains 7.5 mg of methotrexate.
1 pre-filled syringe with 0.5 ml of solution contains 10 mg of methotrexate.
1 pre-filled syringe with 0.625 ml of solution contains 12.5 mg of methotrexate.
1 pre-filled syringe with 0.75 ml of solution contains 15 mg of methotrexate.
1 pre-filled syringe with 0.875 ml of solution contains 17.5 mg of methotrexate.
1 pre-filled syringe with 1 ml of solution contains 20 mg of methotrexate.
1 pre-filled syringe with 1.125 ml of solution contains 22.5 mg of methotrexate.
1 pre-filled syringe with 1.25 ml of solution contains 25 mg of methotrexate.
1 pre-filled syringe with 1.375 ml of solution contains 27.5 mg of methotrexate.
1 pre-filled syringe with 1.5 ml of solution contains 30 mg of methotrexate.
The other ingredients are sodium chloride, sodium hydroxide for pH adjustment, and water for injections.
What Ebetrexat looks like and contents of the pack
Ebetrexat is a clear, yellowish solution for injection in pre-filled syringes.
Each carton contains 1 pre-filled syringe with 0.375 ml, 0.5 ml, 0.625 ml, 0.75 ml, 0.875 ml, 1 ml, 1.125 ml, 1.25 ml, 1.375 ml, or 1.5 ml of solution for injection, a needle for single use with a protective shield or without a shield, and an alcohol swab.
Bundle packs contain 4, 5, 6, 12, or 30 pre-filled syringes (1 pre-filled syringe per carton).
Marketing authorization holder and manufacturer
Marketing authorization holder
Ebewe Pharma Ges.m.b.H. Nfg. KG
Mondseestrasse 11
4866 Unterach, Austria
Manufacturer
Ebewe Pharma Ges.m.b.H. Nfg. KG
Mondseestrasse 11
4866 Unterach, Austria
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
D-39179 Barleben, Germany
Fareva Unterach GmbH
Mondseestraße 11
4866 Unterach, Austria
For more detailed information on the medicine and its names in other EU member states, please contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
tel. 22 209 70 00
Date of last revision of the leaflet:09/2024
Information intended for healthcare professionals only
Ebetrexat, 20 mg/ml, solution for injection in a pre-filled syringe
Instructions for use, handling, and disposal
The solution should be clear and free of particles.
Handling and disposal should be in accordance with national regulations for the preparation of other cytotoxic medicines. Healthcare workers who are pregnant should not handle or administer Ebetrexat.
The medicine is intended for single use only. Any remaining solution should be disposed of.
All unused product or waste materials should be disposed of in accordance with local regulations for cytotoxic medicines.
Incompatibilities
Compatibility with other products has not been studied, so this medicinal product should not be mixed with other medicinal products.
Special precautions for storage
Store in the outer packaging to protect from light.
Do not store above 25°C.
Instructions for subcutaneous injection, step by step
Step 1
- Remove the inner packaging with the pre-filled syringe and needle from the carton.
- Open the inner packaging by pulling the side flap. Remove the pre-filled syringe.
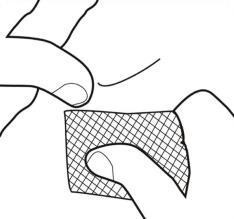
- Remove the gray rubber cap (covered with plastic) from the syringe, without touching the exposed end of the pre-filled syringe (see Figure 1).
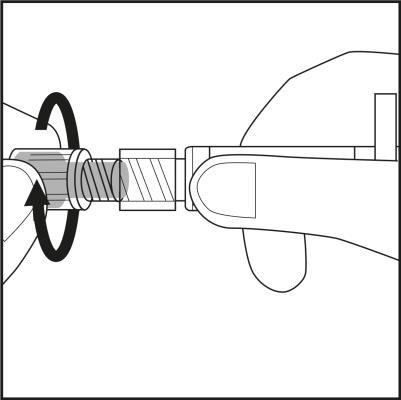
Figure 1.
Step 2
- Put the pre-filled syringe back into the inner packaging. The yellow solution will not leak from the syringe.
- Check the label on the plastic needle shield. The label should not be damaged (see Figure 2)
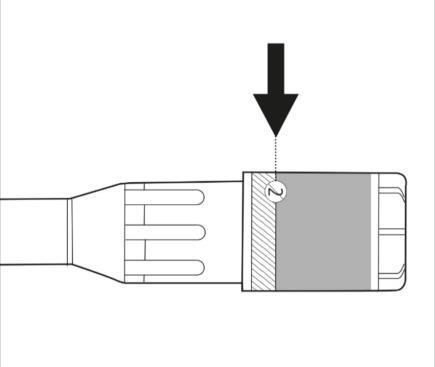
Figure 2.
Step 3.
- Remove the needle cap by twisting and pulling it. See Figure 3.1.
- Carefully screw the needle with its plastic shield onto the syringe until it stops. See Figure 3.2.
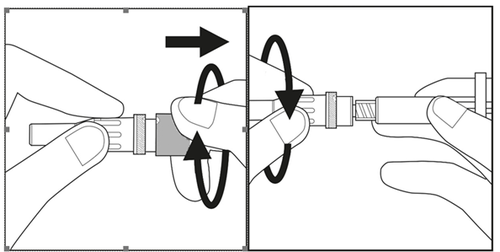
Figure 3.1
Figure 3.2
Step 4
- Choose an injection site on the abdomen or thigh and clean it with an alcohol swab. Do not touch the disinfected injection site (see Figures 4.1 and 4.2).
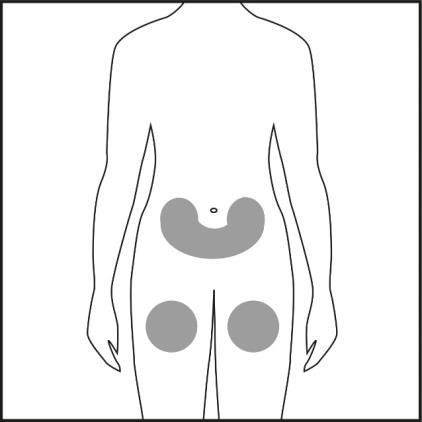
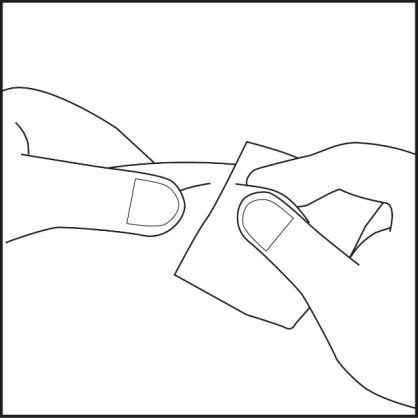
Figure 4.1
Figure 4.2
Step 5.
- Remove the needle shield and set it aside.
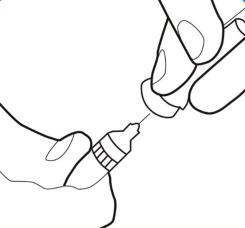
- Do not touch the sterile needle. If you do, ask your doctor or pharmacist for a new needle. Holding the skin with two fingers, create a skin fold and insert the pre-filled syringe needle almost vertically.
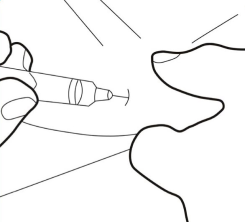
Step 6.
- Insert the needle all the way into the skin fold. Then, slowly press the syringe plunger to inject the entire solution subcutaneously.
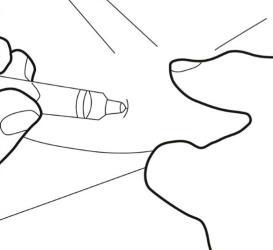
Step 7.
- Carefully pull the needle out of the skin fold and apply a swab to the injection site. Do not rub the injection site, as this may cause irritation.
- To avoid needlestick injury, dispose of the used syringe in a special container.
3. How to use Ebetrexat
Important warning regarding Ebetrexat dosing (methotrexate):
In the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and psoriasis, Ebetrexat should be used only once a week. Using more Ebetrexat (methotrexate) can be fatal. It is very important to carefully read section 3 of this leaflet. If the patient has any questions, they should consult their doctor or pharmacist before taking the medicine.
Ebetrexat can only be prescribed by doctors familiar with the properties of this medicine and its mode of action.
This medicine should always be used exactly as prescribed by the doctor. If the patient has any doubts, they should consult their doctor or pharmacist.
Ebetrexat is administered only once a week. Together with the doctor, the patient should choose a suitable day of the week for the injection.
Improper use of Ebetrexat can lead to severe side effects, which can be fatal.
Typically, the recommended dose is:
Dosage in patients with rheumatoid arthritis
The recommended initial dose is 7.5 mg of methotrexate once a week. Ebetrexat is administered in a single subcutaneous, intramuscular, or intravenous injection (see "Method of administration and duration of treatment").
If Ebetrexat is not sufficiently effective and the patient tolerates it well, the doctor may increase the dose. The average weekly dose of methotrexate is 15-20 mg.
Generally, the weekly dose should not exceed 25 mg. After achieving the desired therapeutic effect, the dose should be gradually reduced to the smallest effective maintenance dose in the individual patient.
Dosage in children and adolescents under 16 years of age with polyarticular forms of juvenile idiopathic arthritis
The recommended dose is 10-15 mg/m2 body surface area per week. If the response to treatment is insufficient, the doctor may increase the weekly dose to 20 mg/m2 body surface area per week. More frequent monitoring is then necessary. Due to the limited amount of data on intravenous administration in children and adolescents, methotrexate should only be administered subcutaneously and intramuscularly.
The use of the medicine is not recommended in children under 3 years of age due to insufficient experience in this age group.
Adults with severe psoriasis or psoriatic arthritis
A single test dose of 5-10 mg is recommended to assess potential toxic effects. This dose can be administered subcutaneously, intramuscularly, or intravenously.
If no changes in blood cell count occur after one week, treatment can be continued with a dose of approximately 7.5 mg. This dose can be gradually increased (by 5-7.5 mg per week, monitoring blood cell count) to achieve optimal treatment results. A weekly dose of 20 mg may be associated with a significant increase in toxicity. The dose should not exceed 30 mg per week.
After achieving the desired therapeutic effect, the dose should be reduced weekly to the smallest effective maintenance dose in the individual patient.
Patients with kidney function disorders
In patients with kidney function disorders, it may be necessary to reduce the dose of the medicine.
Method of administration and duration of treatment
The duration of treatment is determined by the treating doctor. Ebetrexat is used in an injection once a week!It is recommended to choose one day of the week for the injection. Ebetrexat is administered subcutaneously, intramuscularly, or intravenously. Children and adolescents should not be administered the medicine intravenously.
Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, and psoriatic arthritis requires long-term use of Ebetrexat.
Rheumatoid arthritis
Improvement in symptoms is usually observed after 4-8 weeks of treatment. After discontinuation of Ebetrexat, symptoms may recur.
Severe forms of psoriasis and psoriatic arthritis (psoriasis arthropatica)
The expected response to treatment usually occurs after 2-6 weeks.
Depending on the severity of symptoms and laboratory values, treatment should be continued or discontinued.
Initially, Ebetrexat may be administered by medical personnel. However, the doctor may decide that the patient is able to self-administer Ebetrexat injections. The patient will be properly trained in this regard. The patient should never attempt to self-administer the injection without prior training.
Further information can be found in the "Instructions for using Ebetrexat in a pre-filled syringe" section.
Using a higher dose of Ebetrexat than recommended
The patient should use the dose prescribed by their doctor. The patient should never change the dose themselves.
If it is suspected that the patient (or someone else) has used a higher dose of the medicine than they should have, they should immediately contact a doctor or the emergency department of the nearest hospital. Only a doctor can decide on the type of treatment to be undertaken, depending on the severity of the poisoning symptoms.
Methotrexate overdose can cause severe toxic reactions. Symptoms of overdose include easy bruising or bleeding, unexplained weakness, mouth sores, nausea, vomiting, black or bloody stools, coughing up blood or vomiting coffee grounds-like material, and decreased urine output. See also section 4.
Patient should bring the medicine packaging with them to the hospital or doctor.
The antidote used in case of poisoning is calcium folinate.
Missing a dose of Ebetrexat
The patient should not take a double dose to make up for a missed dose. The patient should continue taking the recommended dose of the medicine. The patient should consult their doctor for advice.
Stopping treatment with Ebetrexat
The patient should not stop or discontinue treatment with Ebetrexat without consulting their doctor.
If the patient suspects severe side effects, they should immediately consult their doctor.
If the patient has any further questions or concerns about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The patient should immediately inform their doctor if they experience sudden wheezing, difficulty breathing, swelling of the eyelids, face, or lips, rash, or itching (especially if it affects the whole body) and fainting (these may be symptoms of severe allergic reactions or anaphylactic shock).
Severe side effects
The patient should immediately contact their doctor if they experience any of the following side effects:
- respiratory problems (symptoms may include general malaise; dry, irritating cough; shortness of breath, difficulty breathing at rest, chest pain, and fever);
- hemoptysis, i.e. coughing up sputum with blood;
- severe skin peeling or blistering (also in the mouth, eyes, and genital areas);
- unexplained bleeding (including bloody vomiting) or bruising;
- severe diarrhea;
- mouth ulcers;
- black or bloody stools;
- blood in urine or stool;
- small red spots on the skin;
- fever;
- jaundice (yellowing of the skin);
- pain or difficulty urinating;
- feeling thirsty and/or frequent urination;
- seizures (convulsions);
- loss of consciousness;
- blurred vision or vision disturbances.
Other side effects have also been reported:
Very common (may affect more than 1 in 10 people):
- loss of appetite, nausea, vomiting, abdominal pain
- inflammation and ulcers of the mouth and throat
- increased liver enzyme activity.
Common (may affect up to 1 in 10 people):
- decreased production of blood cells with decreased white blood cell and/or red blood cell or platelet count (leukopenia, anemia, thrombocytopenia)
- headache
- feeling tired, drowsy
- tingling, prickling, or burning sensation of the skin, rash, redness of the skin, itching
- pneumonia
- diarrhea.
Uncommon (may affect up to 1 in 100 people):
- shingles
- lymphoma (which may resolve after discontinuation of the medicine)
- decreased blood cell and platelet count
- severe allergic reactions
- diabetes
- depression
- muscle weakness on one side of the body
- dizziness, disorientation
- seizures
- brain damage (leukoencephalopathy/encephalopathy)
- vasculitis
- lung damage, fluid in the space around the lungs
- ulcers and bleeding in the digestive tract
- pancreatitis
- liver function disorders
- decreased protein levels in the blood
- hives (without other symptoms), increased sensitivity of the skin to sunlight, brown discoloration of the skin
- severe toxic skin reactions, including blistering and peeling (Stevens-Johnson syndrome, Lyell syndrome)
- hair loss
- enlargement of rheumatoid nodules
- painful psoriatic lesions
- reactions resembling sunburn due to increased skin sensitivity to sunlight
- joint or muscle pain
- osteoporosis (decreased bone mass)
- inflammation and ulcers of the bladder (which may be accompanied by blood in the urine), painful urination
- birth defects in the fetus
- inflammation and ulcers of the vagina
- burning or damage to tissue after intramuscular injection.
Rare (may affect up to 1 in 1,000 people):
- sepsis
- presence of very large red blood cells in the blood (megaloblastic anemia)
- mood swings
- transient perceptual disturbances
- weakness of voluntary muscle movements
- speech difficulties
- severe eye disorders
- low blood pressure
- blood clots
- sore throat
- respiratory arrest
- inflammation of the digestive tract, bloody stools
- gingivitis
- acute hepatitis
- change in nail color, nail loss
- acne, presence of red or purple spots due to bleeding
- stress fractures
- electrolyte disturbances
- miscarriage
- abnormal sperm production
- menstrual disorders.
Very rare (may affect up to 1 in 10,000 people):
- herpes labialis
- hepatitis
- severe bone marrow failure
- immunodeficiency (hypogammaglobulinemia)
- pain
- muscle weakness
- taste disturbances (metallic taste in the mouth)
- meningitis (inflammation of the membranes covering the brain), causing paralysis or vomiting
- eye redness
- pericarditis (inflammation of the "bag" surrounding the heart), fluid in the pericardium
- pneumonia, difficulty breathing, asthma
- bloody vomiting
- liver failure
- paronychia (infection of the nail bed), furuncles, presence of small blood vessels visible on the skin
- protein in the urine
- fetal death
- disorders of egg cell maturation in women and sperm production in men
- loss of libido
- erectile dysfunction
- leukorrhea
- infertility
- mild skin reactions after subcutaneous injection
- lymphoproliferative disorders (overproduction of white blood cells)
- feeling of numbness or tingling or decreased reaction to stimuli.
Frequency not known (cannot be estimated from the available data):
- infections, which can be life-threatening in some cases
- lymph node swelling
- immune system disorders
- fever
- vasculitis due to allergic reaction
- peritonitis (inflammation of the membrane lining the abdominal cavity)
- slow wound healing
- pulmonary hemorrhage
- bone damage in the jaw (due to overproduction of white blood cells)
- tissue destruction at the injection site
- redness and peeling of the skin
- edema.
After intramuscular administration, side effects (burning sensation) or tissue damage (formation of a sterile abscess, fat tissue atrophy) at the injection site may occur frequently. Subcutaneous administration of methotrexate is well tolerated. Only mild skin reactions have been observed, which decrease during treatment.
Methotrexate may decrease the production of white blood cells and weaken the immune system against infections. If the patient develops an infection with symptoms such as fever and significant deterioration of general health, or fever and symptoms of local infection (sore throat and/or pharyngitis), or urinary disorders, they should immediately consult their doctor. The doctor will order blood tests to determine if the patient has a decreased white blood cell count (agranulocytosis). It is essential to inform the doctor about the use of Ebetrexat.
Methotrexate may cause severe (sometimes life-threatening) side effects. Therefore, the doctor will order tests to detect potential changes in the blood picture (e.g. decreased white blood cell count, low platelet count, presence of lymphoma) and changes in the kidneys and liver.
Reporting side effects
If the patient experiences any side effects, including those not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products of the Office for Registration of Medicinal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects helps to gather more information on the safety of the medicine.
5. How to store Ebetrexat
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the label of the pre-filled syringe and on the carton after EXP. The expiry date refers to the last day of the month.
The pre-filled syringe should be stored in the outer packaging to protect it from light.
The medicine should not be stored above 25°C.
The medicine should be used immediately after opening.
The patient should not use Ebetrexat if the solution is not clear and contains precipitated particles.
The medicine is for single use only. Any remaining solution should be disposed of!
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Ebetrexat contains
The active substance is methotrexate.
Each ml of solution for injection contains 20 mg of methotrexate (as 21.94 mg of methotrexate disodium)
Each pre-filled syringe with 0.375 ml of solution contains 7.5 mg of methotrexate.
Each pre-filled syringe with 0.5 ml of solution contains 10 mg of methotrexate.
Each pre-filled syringe with 0.625 ml of solution contains 12.5 mg of methotrexate.
Each pre-filled syringe with 0.75 ml of solution contains 15 mg of methotrexate.
Each pre-filled syringe with 0.875 ml of solution contains 17.5 mg of methotrexate.
Each pre-filled syringe with 1 ml of solution contains 20 mg of methotrexate.
Each pre-filled syringe with 1.125 ml of solution contains 22.5 mg of methotrexate.
Each pre-filled syringe with 1.25 ml of solution contains 25 mg of methotrexate.
Each pre-filled syringe with 1.375 ml of solution contains 27.5 mg of methotrexate.
Each pre-filled syringe with 1.5 ml of solution contains 30 mg of methotrexate.
The other ingredients are sodium chloride, sodium hydroxide for pH adjustment, and water for injections.
What Ebetrexat looks like and contents of the pack
Ebetrexat is a clear, yellowish solution for injection in pre-filled syringes.
Each carton contains 1 pre-filled syringe with 0.375 ml, 0.5 ml, 0.625 ml, 0.75 ml, 0.875 ml, 1 ml, 1.125 ml, 1.25 ml, 1.375 ml, or 1.5 ml of solution for injection, a needle with a protective shield or without a shield, and an alcohol swab.
Bulk packs contain 4, 5, 6, 12, or 30 pre-filled syringes (1 pre-filled syringe per carton).
Marketing authorization holder and manufacturer
Marketing authorization holder
Ebewe Pharma Ges.m.b.H. Nfg. KG
Mondseestrasse 11
4866 Unterach, Austria
Manufacturer
Ebewe Pharma Ges.m.b.H. Nfg. KG
Mondseestrasse 11
4866 Unterach, Austria
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
D-39179 Barleben, Germany
Fareva Unterach GmbH
Mondseestraße 11
4866 Unterach, Austria
For more information about the medicine and its names in other EU countries, please contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warszawa
tel. 22 209 70 00
Date of last revision of the leaflet:09/2024
Instructions for using Ebetrexat in a pre-filled syringe
The solution should be yellow and clear. It should not be mixed with other medicinal products.
Handling and disposal should be in accordance with national regulations for the preparation of other cytotoxic drugs. Pregnant women, including healthcare workers who are pregnant, should not handle or administer Ebetrexat.
To use the pre-filled syringe correctly, the patient should follow the instructions for injection step by step:
Step 1
- Remove the pre-filled syringe from the carton and take out the inner packaging.
- Open the inner packaging by pulling the side flap. Remove the pre-filled syringe.
- Remove the gray rubber cap (covered with plastic) from the syringe, without touching the exposed end of the pre-filled syringe (see Figure 1).
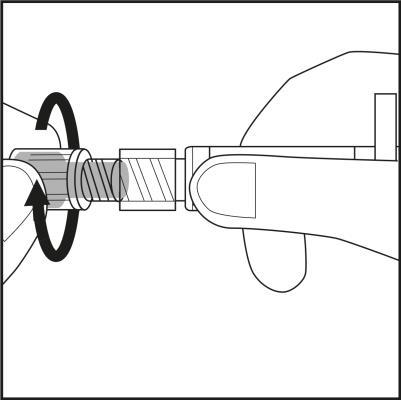
Figure 1.
Step 2
- Put the syringe back into the inner packaging. The yellow solution will not leak from the syringe.
- Check the label on the plastic needle shield. The label must not be damaged! (see Figure 2)
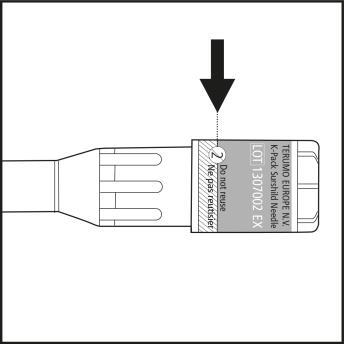
Figure 2.
Step 3
- Remove the needle cap from the plastic needle shield by twisting and pulling. See Figure 3.1.
- Carefully screw the needle with its plastic shield onto the syringe until it stops. See Figure 3.2.
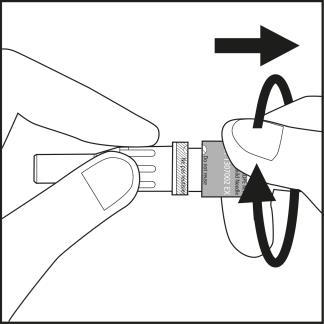
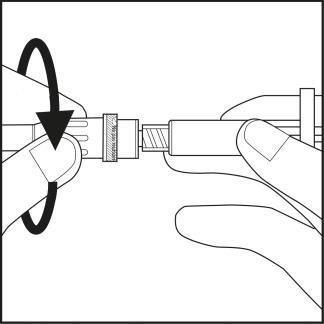
Figure 3.1
Figure 3.2
Step 4
- Choose an injection site on the abdomen or thigh and clean it with an alcohol swab. Do not touch the disinfected injection site (see Figures 4.1 and 4.2).
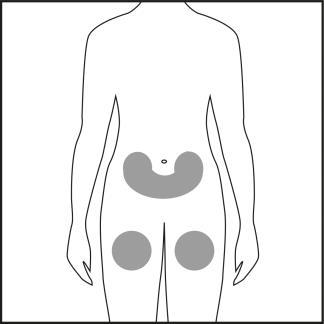
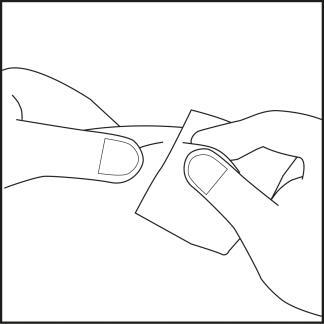
Figure 4.1
Figure 4.2
Step 5
- Pull the shield straight back to remove it from the needle. Check that the indicator has a blue color (see arrow on Figure 5.1).
- The syringe is now ready for use. Caution:The needle shield should not be put back on the needle to avoid accidentally triggering the safety mechanism. The needle shield should not be touched before injection (see Figure 5.2). Any pressure on the shield may cause the safety mechanism to close and render the needle unusable. In this case, the blue indicator will not appear in the window.
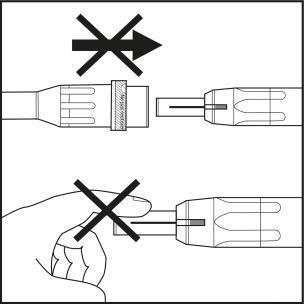
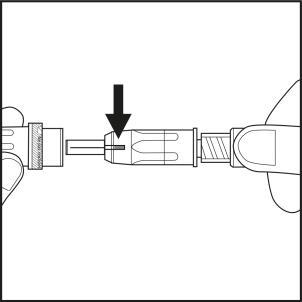
Figure 5.1
Figure 5.2
Step 6
- Pinch the skin at the chosen injection site to form a fold, using two fingers.
- Hold the syringe with the other hand as shown in Figure 6.1.
- Firmly, with one continuous motion, insert the needle into the skin fold at a nearly vertical angle. Insert the needle until the shield is fully retracted! See Figure 6.2.
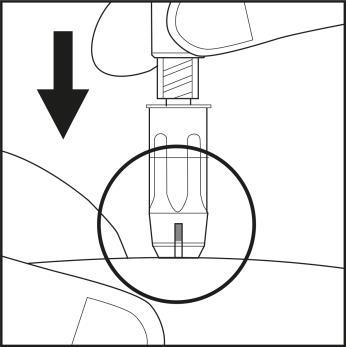
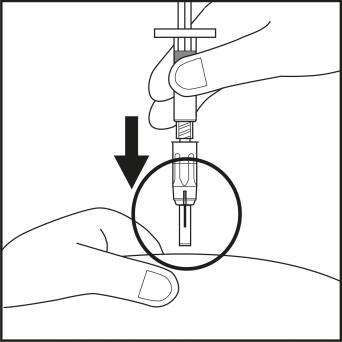
Figure 6.1
Figure 6.2
- Maintaining steady pressure on the syringe, slowly press the plunger to inject the solution under the skin (see Figure 6.3).
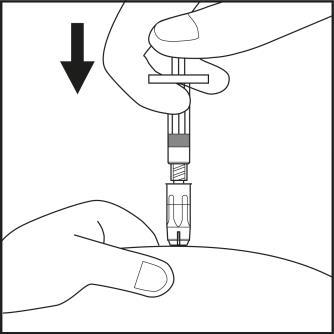
Figure 6.3
Step 7
- After injecting the medicine, the blue indicator will disappear from the window, confirming that the safety mechanism has been activated automatically. The needle shield is activated and protects against needlestick injury (see Figure 7).
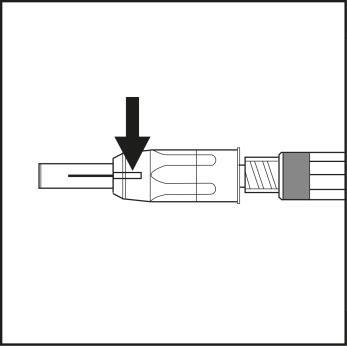
Figure 7
- Press a swab against the injection site until bleeding stops. Do not rub the skin to avoid irritating the injection site.
- Dispose of the used syringe and needle in a special container intended for this purpose.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterEbewe Pharma Ges.m.b.H Nfg. KG Fareva Unterach GmbH Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EbetrexatDosage form: Solution, 7.5 mgActive substance: methotrexatePrescription requiredDosage form: Solution, 12.5 mgActive substance: methotrexatePrescription requiredDosage form: Solution, 10 mgActive substance: methotrexatePrescription required
Alternatives to Ebetrexat in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ebetrexat in Іспанія
Alternative to Ebetrexat in Україна
Online doctors for Ebetrexat
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ebetrexat – subject to medical assessment and local rules.






