How to use Loxon 2%
Package Leaflet: Information for the Patient
LOXON 2%
Minoxidil
20 mg/ml, liquid for the skin
{logo of the responsible entity}
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or according to the doctor's or pharmacist's instructions.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, the pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. See section 4.
- If there is no improvement or the patient feels worse, the doctor should be contacted.
Table of Contents of the Leaflet
- 1. What is Loxon 2% and what is it used for
- 2. Important information before using Loxon 2%
- 3. How to use Loxon 2%
- 4. Possible side effects
- 5. How to store Loxon 2%
- 6. Contents of the pack and other information
1. What is Loxon 2% and what is it used for
Loxon 2% is a liquid for application to the scalp and contains the active substance minoxidil. Minoxidil stimulates hair growth in androgenetic alopecia in women and men. It reverses or slows down the miniaturization of hair follicles, strongly stimulating the proliferation of hair follicle cells in the growth phase. Hair loss cessation has been observed in the second month of use, and hair growth usually begins after 4 months of treatment with Loxon 2%. The maximum effect is achieved after about 12 months of systematic use, and the treatment results are largely dependent on systematic use of the medicine and individual patient characteristics.
2. Important information before using Loxon 2%
When not to use Loxon 2%
Warnings and precautions
Before starting to use Loxon 2%, the doctor or pharmacist should be consulted. The medicine is intended for use on the scalp only; it should not be used on damaged skin (inflammatory conditions, psoriasis) or shaved scalp. Loxon 2% should not be used during the use of other topical medicines. Topical medicines, such as corticosteroids, tretinoin, ditranol, and petroleum jelly, may alter the protective properties of the stratum corneum, leading to increased absorption of minoxidil into the blood and its systemic action. The use of Loxon 2% should be discontinued in case of hypotension, chest pain, accelerated heart rate, fainting, dizziness, or limb swelling. In case of persistent redness of the scalp or irritation, the use of the medicine should be discontinued and the doctor consulted. Patients with cardiovascular diseases or rhythm disorders should consult their doctor before starting treatment. At the beginning of treatment (usually 2-6 weeks), increased hair loss may occur. If increased hair loss persists for more than 2 weeks, the use of Loxon 2% should be discontinued and the doctor consulted. Inhaling the medicine should be avoided. There have been cases of excessive hair growth on the body of infants following skin contact with minoxidil application sites in patients (caregivers) using minoxidil topically. Hair growth returned to normal within a few months when the infant was no longer exposed to minoxidil. Children should be kept away from areas of the body where minoxidil has been applied topically. If excessive hair growth on the child's body is observed during the use of minoxidil-containing products, the doctor should be consulted.
Loxon 2% and other medicines
The doctor or pharmacist should be informed about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to use.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant, or plans to have a child, she should consult her doctor or pharmacist before using this medicine.
Pregnancy
The medicine should not be used during pregnancy.
Breastfeeding
The medicine should not be used during breastfeeding.
Driving and using machines
The medicine does not affect the patient's ability to drive or use machines.
Loxon 2% contains ethanol 96%
This medicine contains 500 mg of alcohol (ethanol) in 1 ml of liquid, which is one dose (average volume of 10 sprays).
One spray of 0.1 ml of liquid contains 50 mg of ethanol.
The medicine contains alcohol, which may irritate the mucous membranes and eyes; in case of the medicine getting into the eye, it should be rinsed thoroughly with water.
The medicine may cause burning of damaged skin.
The medicine is flammable. It should not be used near open fire, lit cigarette, or electrical appliances, such as a hair dryer.
Loxon 2% contains propylene glycol
The medicine contains 300 mg of propylene glycol in each ml of liquid.
Propylene glycol may cause skin irritation.
3. How to use Loxon 2%
This medicine should always be used exactly as described in the patient leaflet or according to the doctor's or pharmacist's instructions. In case of doubts, the doctor or pharmacist should be consulted.
The medicine is used twice a day.
1 ml of liquid should be applied to the balding area and massaged into the skin (usually the top of the head and the frontal area).
The volume of 1 spray is 0.1 ml.
The average volume of 10 sprays is 1 ml.
The dose should not exceed 2 ml per day.
Note: After applying the liquid to the scalp, hands should be washed thoroughly.
Treatment should be discontinued if there is no improvement after 1 year of use.
Use in children and adolescents
The medicine should not be used in children under 18 years old.
If it seems that the effect of Loxon 2% is too strong or too weak, the doctor should be consulted.
Instructions for using the bottle with a dispenser
Before use, turn the applicator to the side and then press the dispenser to apply the medicine to the scalp (balding area).
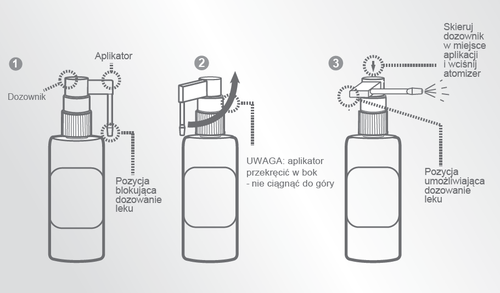
WARNING - PROTECT EYES, NOSE, AND MOUTH.
Using a higher dose of Loxon 2% than recommended
There are no known cases of minoxidil overdose when used topically.
Due to the high concentration of minoxidil in Loxon 2%, accidental ingestion of the medicine may cause symptoms related to its systemic action. The most common symptoms include: tachycardia (accelerated heart rate), fainting, dizziness, sudden weight gain.
If such symptoms are observed, the doctor should be contacted immediately.
Missing a dose of Loxon 2%
In case of missing one application of the medicine, it should be applied to the skin as soon as possible, unless it is close to the time of the next application.
A double dose should not be used to make up for the missed dose of Loxon 2%.
4. Possible side effects
The doctor should be contacted immediately if any of the following symptoms occur, as urgent medical attention may be necessary:
- Swelling of the face, lips, or throat, which makes swallowing or breathing difficult. These may be symptoms of a severe allergic reaction (frequency not known, cannot be estimated from available data).
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Rarely(in 1 to 10 out of 10,000 patients): skin reactions, such as skin irritation and itching, excessive hair growth (including the appearance of facial hair in women), local redness, drying/scaling of the scalp, and increased hair loss.
Rarecases of hypotension.
Reporting side effects
If any side effects occur, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Loxon 2%
The medicine should be stored at a temperature below 25°C.
The medicine should be kept out of sight and reach of children.
The medicine should not be used after the expiry date stated on the packaging.
The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of unused medicines. This will help protect the environment.
6. Contents of the pack and other information
What Loxon 2% contains
The active substance of the medicine is minoxidil.
1 ml of liquid for the skin contains 20 mg of minoxidil.
The excipients are: propylene glycol, ethanol 96%, purified water, citric acid.
What Loxon 2% looks like and contents of the pack
Loxon 2% is a liquid for the skin.
Packaging:60 ml of liquid for the skin in a high-density polyethylene (HDPE) bottle with a pump [low-density polyethylene (LDPE), high-density polyethylene (HDPE), polypropylene (PP), stainless steel, ethylene-vinyl acetate (EVA), polyoxymethylene (POM)] with a dispenser [high-density polyethylene (HDPE), polypropylene (PP)] or with a pump [low-density polyethylene (LDPE), polypropylene (PP)] with a dispenser [polypropylene (PP)], placed in a cardboard box.
Marketing authorization holder and manufacturer
Marketing authorization holder
Perrigo Poland Sp. z o.o.
ul. Domaniewska 48
02-672 Warsaw
+48 (22) 852 55 51
Manufacturer
Opella Healthcare Poland Sp. z o.o.
Branch in Rzeszów
ul. Lubelska 52
35-233 Rzeszów
To obtain more detailed information about the medicine, please contact:
Perrigo Poland Sp. z o.o.
ul. Domaniewska 48
02-672 Warsaw
+48 (22) 852 55 51
Date of leaflet approval:October 2024
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterOpella Healthcare Poland Sp. z o.o. Oddział w Rzeszowie
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Loxon 2%Dosage form: Solution, 20 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Solution, 50 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Aerosol, 50 mg/mlActive substance: minoxidilManufacturer: mibe GmbH Arzneimittel Sun-Farm Sp. z o.o.Prescription not required
Alternatives to Loxon 2% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Loxon 2% in Ukraine
Alternative to Loxon 2% in Spain
Online doctors for Loxon 2%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Loxon 2% – subject to medical assessment and local rules.