

Lidbree

Ask a doctor about a prescription for Lidbree

How to use Lidbree
Package Leaflet: Information for the User
Lidbree, 42 mg/ml, Intrauterine Gel
Lidocaine
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What is Lidbree and what is it used for
- 2. Important information before using Lidbree
- 3. How to use Lidbree
- 4. Possible side effects
- 5. How to store Lidbree
- 6. Contents of the pack and other information
1. What is Lidbree and what is it used for
Lidbree is an anesthetic gel used to prevent pain during gynecological procedures, such as the insertion of intrauterine contraceptives and the collection of biopsy samples for laboratory evaluation in gynecological examinations, in adults and adolescents over 15 years of age. It contains the active substance lidocaine, a local anesthetic (numbs the parts of the body to which it is applied).
How Lidbree works
After application of the gel, it takes from 2 to 5 minutes for the genital area (mucous membrane) to become numb. The gel has been shown to reduce pain during gynecological procedures and for at least 30 minutes after the procedure. The pain-relieving effect disappears after 1 hour.
2. Important information before using Lidbree
When not to use Lidbree:
- if you are allergic to lidocaine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
For intrauterine use only. After applying the gel before inserting intrauterine contraceptives (intrauterine contraceptive devices), bleeding and/or excessive pain may occur in some cases. In such cases, a physical examination and ultrasound examination should be performed immediately to rule out uterine or cervical perforation. It has been found that on average, 1 in 1,000 intrauterine device insertions causes perforation.
Tell the person administering Lidbree to you:
- If you have an irregular heartbeat (partial or complete conduction block in the heart muscle), as local anesthetics may affect it.
- If you are being treated for irregular heart rhythms (so-called potassium channel inhibitors or class III antiarrhythmic drugs, such as amiodarone), as cardiac effects may be enhanced.
- If you have acute porphyria (a family-related condition associated with one of the blood proteins). Lidocaine may cause porphyria attacks and should only be prescribed to patients with acute porphyria in severe or emergency situations.
- If you are in poor health.
Children and adolescents
Children under 15 years of age should not receive this medicine due to the risk of adverse reactions caused by high lidocaine levels in the blood.
Lidbree and other medicines
Tell your doctor or medical staff if you have recently taken any other medicines containing lidocaine or antiarrhythmic drugs (such as mexiletine or class III antiarrhythmic drugs, such as amiodarone), as their cardiac effects may be increased.
Pregnancy and breastfeeding
Based on long-term experience, it is not known that the use of lidocaine during pregnancy has adverse effects on the newborn.
Lidocaine may pass into breast milk, but in such small amounts that it is generally not a risk to the breastfed infant. Therefore, breastfeeding can be continued during treatment with Lidbree.
It is not known whether lidocaine has a negative effect on fertility.
Driving and using machines
Lidbree has no or negligible influence on the ability to drive and use machines.
Lidbree contains macrogolglycerol ricinoleate (polyoxyethylene castor oil) and butylhydroxytoluene (E 321).
Macrogolglycerol ricinoleate may cause severe allergic reactions.
Butylhydroxytoluene (E 321) may cause mucous membrane irritation.
3. How to use Lidbree
The anesthetic gel will be applied by your doctor or nurse, gradually, starting from the entrance to the uterus.
Use in adolescents
Adolescents with a low body weight, below 30 kg, should receive a reduced dose.
Use of a higher than recommended dose of Lidbree
During the use of recommended doses, this is not expected, but if you experience numbness of the lips or tongue, a feeling of emptiness in the head, ringing in the ears (tinnitus) or difficulty speaking or seeing (vision disturbances), you should immediately tell your doctor or nurse, as these may be the first signs of high lidocaine levels in the blood. Sometimes, muscle spasms or tremors, or respiratory arrest (apnea), may occur, in which case the doctor or nurse should immediately ensure adequate breathing (respiratory support) and administer anticonvulsant drugs.
4. Possible side effects
Like all medicines, Lidbree can cause side effects, although not everybody gets them.
The side effects that occur after using Lidbree before inserting intrauterine contraceptives are similar to those that occur when inserting contraceptives without prior use of Lidbree.
Possible side effects are:
- Very common side effects(more than 1 in 10 people): nausea (vomiting).
- Common side effects(may occur in up to 1 in 10 people): dizziness, headache, unpleasant abdominal sensations.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or nurse. Side effects can be reported directly to the Department of Adverse Reactions Monitoring of Medicinal Products, Medical Devices, and Biocidal Products,
Al. Jerozolimskie 181C,
02-222 Warsaw,
tel.: +48 22 49 21 301,
fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Lidbree
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date (month-year) stated on the carton and syringe. The expiry date refers to the last day of the stated month.
There are no special precautions for storage.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
- The active substance is lidocaine. Each ml of intrauterine gel contains 42 mg of lidocaine.
- The other ingredients are:
- Macrogolglycerol ricinoleate (polyoxyethylene castor oil)
- Poloxamer (contains butylhydroxytoluene (E 321))
- Sodium ascorbate (E 301)
- Concentrated hydrochloric acid (for pH adjustment)
- Sodium hydroxide (for pH adjustment)
- Water for injections
What Lidbree looks like and contents of the pack
The medicine is an intrauterine gel (for the uterus), a clear or almost clear, slightly brownish-yellow, viscous liquid at room temperature containing 42 mg/ml of lidocaine. Lidbree exhibits a reversible temperature-dependent change from a liquid to a gel. At body temperature, it is a gel (thermogelation).
Marketing authorization holder
Gedeon Richter Plc.
Gyömrői út 19-21
1103 Budapest
Hungary
Manufacturer
Gedeon Richter Plc.
Gyömrői út 19-21
1103 Budapest
Hungary
Recipharm Karlskoga AB
Björkbornsvägen 5
Karlskoga 691 33
Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria:
Lidbree 42 mg/ml Gel zur intrauterinen Anwendung
Belgium:
Lidbree 42 mg/ml gel voor intra-uterien gebruik
Lidbree 42 mg/ml gel intra-utérin
Lidbree 42 mg/ml Gel zur intrauterinen Anwendung
Bulgaria:
Lidbree 42 mg/ml intrauterine gel
Croatia:
Lidbree 42 mg/ml intrauterini gel
Cyprus:
Lidbree
Czech Republic:
Lidbree
Denmark:
Lidbree
Estonia:
Lidbree
Finland:
Lidbree 42 mg/ml Geeli kohtuun
France:
LIDBREE 42 mg/ml gel intra-utérin
Germany:
Lidbree 42 mg/ml Gel zur intrauterinen Anwendung
Greece:
Lidbree
Hungary:
Lidbree 42 mg/ml intrauterin gél
Iceland:
Lidbree
Ireland:
Lidbree
Italy:
Lidbree
Latvia:
Lidbree 42 mg/ml gimdos ertmės gels
Lithuania:
Lidbree 42 mg/ml intrauterīnais gelis
Luxembourg:
Lidbree 42 mg/ml gel intra-utérin
Netherlands:
Lidbree 42 mg/ml gel voor intra-uterien gebruik
Norway:
Lidbree
Poland:
Lidbree
Portugal:
Lidbree 42 mg/ml gel intrauterino
Malta:
Lidbree 42 mg/mL intrauterine gel
Romania:
Lidbree 42 mg/ml gel cu cedera intrauterină
Slovakia:
Lidbree 42 mg/ml intrauterini gel
Slovenia:
Lidbree 42 mg/ml intrauterinný gél
Spain:
Lidbree 42 mg/ml gel intrauterino
Sweden:
Lidbree 42 mg/ml intrauterin gel
United Kingdom:
Lidbree 42 mg/mL intrauterine gel
To obtain more detailed information on the medicine, please contact:
GEDEON RICHTER POLSKA Sp. z o.o.
Medical Department
ul. Ks. J. Poniatowskiego 5
05-825 Grodzisk Mazowiecki
tel. +48 (22)755 96 48
fax: +48 (22) 755 96 24
Date of last revision of the leaflet:
------------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only:
Intrauterine use only.
After using the medicinal product Lidbree, in case of difficulties with inserting intrauterine contraceptives and/or excessive pain or bleeding during or after their insertion, a physical examination and ultrasound examination should be performed immediately to rule out uterine or cervical perforation, as the patient may not feel pain during effective local anesthesia.
Thermogelating form:the medicinal product Lidbree is a thermogelating, preservative-free, viscous anesthetic liquid. The preparation forms a gel when its temperature rises to body temperature, allowing it to adhere to the mucous membrane tissues of the cervical canal and uterine mucous membrane (limiting leakage that would occur with a liquid preparation).
During administration, the medicinal product Lidbree should be in a liquid state. If a gel has formed, the medicinal product should be placed in the refrigerator until it becomes liquid again. A air bubble will be visible in the syringe, which will move when the syringe is tilted.
The product should be administered step by step and the viscous liquid applied using the provided sterile applicator:
- 1) Check the appearance of the syringe by tilting it. The air bubble in the syringe will move when tilted if the product is in a liquid state and ready for use. If the air bubble does not move, the product has formed a gel – it should be placed in the refrigerator until it becomes liquid again.
- 2) Attach the plunger and applicator to the syringe and ensure they are securely connected.

- 3) Expel the air bubble and fill the applicator with the gel, carefully pressing the syringe plunger.
- 4) To determine the contents of the medicinal product Lidbree, use the centimeter scale on the applicator.
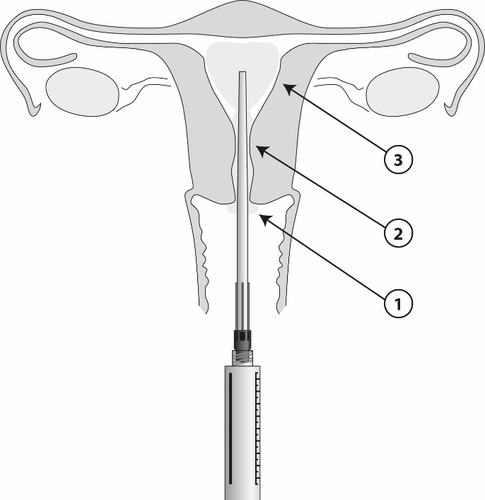
After placing the applicator in the administration site, 8.5 ml of the gel can be administered from the syringe. One ml contains 42 mg of lidocaine. The gel should be administered gradually (from 1 to 3), as shown in the diagram.

Cervical procedures
- 1) Apply 2 to 3 ml of a thick layer to the vaginal part of the cervix using the sterile applicator.
- 2) Apply 3 ml to the cervical canal 5 minutes before the start of the procedure using the sterile applicator.
Intrauterine procedures
- 1) Apply 1 to 2 ml to the anterior lip of the cervix using the sterile applicator.
- 2) Administer 2 to 3 ml to the cervical canal using the applicator. Wait 2 minutes for the effect to appear in the inner muscle tissue.
- 3) Then, insert the applicator into the uterine cavity and administer 3 to 5 ml 5 minutes before the procedure. The applicator is marked with a centimeter scale. A smaller volume may be administered, for example, to patients who have never given birth, if the patient experiences discomfort before administering the full volume.
A single intrauterine dose should not exceed 10 ml in total. Any unused items should be discarded.
Children and adolescents over 15 years of age
In adolescents with a low body weight, below 30 kg, the dose should be proportionally reduced, and a single dose should not exceed the maximum recommended dose (6 mg/kg body weight of lidocaine hydrochloride, which corresponds to 5.2 mg/kg body weight of lidocaine contained in the medicinal product Lidbree, i.e., 1.2 ml per 10 kg body weight). In adolescents with a body weight of 30 kg, the maximum dose of the medicinal product Lidbree is 3.6 ml in total.
Duration of effect
It has been shown that the gel reduces pain during gynecological procedures and for at least 30 minutes after the procedure. The pain-relieving effect disappears after 1 hour.
 |  |  |  |  |
| Catalog number | Batch number | Do not use if the packaging is damaged | Do not reuse | CE marking |
    |  |  |  | |
| Manufacturer | Use before the expiry date | Sterilized by irradiation | Read the instructions for use |
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterGedeon Richter Plc. Recipharm Karlskoga AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LidbreeDosage form: Gel, 0.5 g/100 gActive substance: lidocainePrescription not requiredDosage form: Aerosol, 96 mg/gActive substance: lidocaineManufacturer: Aflofarm Farmacja Polska Sp. z o.o.Prescription not requiredDosage form: Gel, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler Co.GmbHPrescription not required
Alternatives to Lidbree in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lidbree in Spain
Alternative to Lidbree in Ukraine
Online doctors for Lidbree
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lidbree – subject to medical assessment and local rules.














