
Levomer
Ask a doctor about a prescription for Levomer

How to use Levomer
Package Leaflet: Information for the User
Levomer, 5 mg/ml, Eye Drops, Solution
Levofloxacin (in the form of hemihydrate)
Read the package leaflet carefully before using the medicine, as it contains
important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What is Levomer eye drops, solution and what is it used for
- 2. Important information before using Levomer eye drops, solution
- 3. How to use Levomer eye drops, solution
- 4. Possible side effects
- 5. How to store Levomer eye drops, solution
- 6. Contents of the pack and other information
1. What is Levomer eye drops, solution and what is it used for
One bottle of Levomer 5 mg/ml eye drops contains 5 ml of solution containing the active substance levofloxacin.
In one drop corresponding to 0.05 ml of the medicine, there is 0.256 mg of levofloxacin hemihydrate, which corresponds to 0.250 mg of levofloxacin.
Levofloxacin is an antibiotic from the group called fluoroquinolones (sometimes abbreviated as quinolones). The action of the medicine is to destroy certain types of bacteria that can cause infections.
Levofloxacin in the form of eye drops is used in children who have completed 1 year of age or older and in adults to treat bacterial infections of the anterior surface of the eye. One type of infection in this area is bacterial conjunctivitis, which is an infection of the superficial layer of the front of the eye (conjunctiva).
Levomer eye drops are not suitable for treating infections of the deeper layers of the eye or the fluid in the eyeball. For the treatment of such deeper and more serious infections, it is necessary to use an antibiotic in tablets or injections.
It is not recommended to use Levomer eye drops in children under one year of age.
If after 5 days there is no improvement or the patient feels worse, they should consult a doctor.
2. Important information before using Levomer eye drops, solution
When not to use Levomer eye drops, solution:
- if the patient is allergic to levofloxacin, other quinolones, or any of the other ingredients of this medicine (listed in section 6);
in case of doubt, consult a doctor or pharmacist.
- if in doubt, consult a doctor or pharmacist.
Warnings and precautions
Before starting to use Levomer eye drops, discuss it with your doctor, pharmacist, or nurse:
- if an allergic reaction occurs, even after a single dose, discontinue the use of the medicine,
- if during treatment the patient observes worsening of eye symptoms, they should contact their doctor as soon as possible,
- if after a certain period of treatment established by the doctor there are no signs of recovery, the patient should contact their doctor as soon as possible,
- usually, during an eye infection, no contact lenses should be worn,
- Levomer eye drops contain a preservative, benzalkonium chloride, which may cause eye irritation.
Swelling and tendon rupture have occurred in people taking fluoroquinolones orally or intravenously, especially in older patients and those treated with corticosteroids at the same time. Treatment with Levomer eye drops should be discontinued if pain or swelling of the tendon (tendinitis) occurs.
Children and adolescents
Special warnings and precautions for use of the medicine are the same for adults, adolescents, and children over one year of age.
Levomer eye drops, solution and other medicines
Tell your doctor or pharmacist about all medicines, including those without a prescription, that the patient is currently taking or has recently taken, as well as about medicines that the patient plans to take.
It is especially important to tell your doctor or pharmacist before starting to use Levomer eye drops about the use of any other eye drops or ointments.
If the patient is using other eye drops, they should wait at least 15 minutes between using Levomer eye drops and other eye drops.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Levomer eye drops may be used in pregnant women only when the expected benefit outweighs the potential risk to the developing child.
Although levofloxacin penetrates into the blood and breast milk in very small amounts after administration of eye drops, it is very unlikely that the eye drops will harm the developing child.
The doctor is informed about the potential risk and will advise the patient whether to use Levomer eye drops in such a situation.
When using Levomer according to the recommendations, fertility (ability to become pregnant or father a child) is not impaired.
Driving and using machines
Levomer has a minor influence on the ability to drive and use machines.
If the eye drops cause blurred vision, wait until your vision clears before driving or using machines.
Important information about some ingredients of Levomer
Levomer contains benzalkonium chloride
The medicine contains 0.05 mg of benzalkonium chloride in each ml of solution, which corresponds to 0.25 mg in 5 ml. Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Contact lenses should be removed before instillation and waited at least 15 minutes before reinsertion. Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or disorders of the cornea (the transparent layer on the front of the eye). If abnormal sensations in the eye, stinging, or pain in the eye occur after using the medicine, consult a doctor.
3. How to use Levomer eye drops, solution
This medicine should always be used exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Levomer eye drops are intended for use in the eye and must be used on the outer surface of the eye. In patients over one year of age, the recommended dose is:
Day 1-2
- One to two drops into the infected eye (eyes) every two hours.
- The medicine should be used no more than eight times a day. Day 3-5
- One to two drops into the infected eye (eyes).
- The medicine should be used no more than four times a day.
There is no need to modify the dose in elderly people.
Treatment usually lasts for five days. The doctor will inform the patient how long to use the drops.
If the patient is using any other eye medicine, they should wait at least 15 minutes between using the other eye drops.
Use in children and adolescents
There is no need to modify the dose in children over one year of age and adolescents. It is not recommended to use Levomer in children under one year of age.
Before administering the drops:
If possible, ask another person to administer the drops. Before administering the drops, ask that person to read these instructions to the patient.
- 1) Wash your hands.
- 2) Open the bottle. Be careful not to touch the tip of the dropper with your eye, the skin around your eye, or your fingers. If this happens, contact your doctor or pharmacist to get a clean bottle. Tilt your head back in a sitting or lying position.

- 3) Bring the tip of the bottle close to the eye.
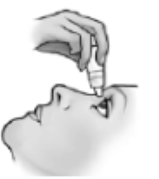
- 4) Pull the lower eyelid down and look up.
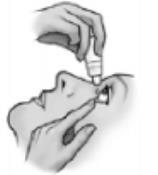
- 5) Gently squeeze the bottle to release one drop into the space between the lower eyelid and the eye.
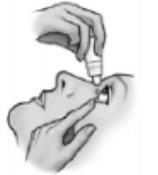
- 6) Close your eye for a moment and press the inner corner of your eye with your index finger for about one minute. This will prevent the drop from flowing through the tear duct.
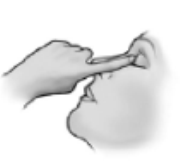
- 7) Wipe any remaining solution from the skin around the eye. If it is necessary to administer another drop, as well as when treating the second eye, repeat the actions described in points 3 to 6. After use, close the bottle tightly.
Levomer must not be injected into the inner part of the eyeball.
Using more than the recommended dose of Levomer eye drops, solution
In case of using more than the recommended dose of Levomer eye drops, flush the eye (eyes) with water and inform your doctor or pharmacist.
Missing a dose of Levomer eye drops, solution
In case of missing a dose of eye drops, use the next dose as soon as you remember. Do not use a double dose to make up for the missed dose.
Accidental ingestion of Levomer eye drops, solution
The amount of levofloxacin contained in the bottle is too small to cause side effects. However, in case of doubt, inform your doctor or pharmacist, who will advise on the necessary measures.
Stopping the use of Levomer eye drops, solution earlier than recommended by the doctormay prolong the healing process.
In case of any further doubts about the use of this medicine, consult your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
They occur in about one in ten people using Levomer eye drops. Most of these side effects are related to the eye and usually do not last long. If the patient experiences any severe or prolonged side effects, they should discontinue the use of Levomer eye drops and seek medical advice immediately.
The frequency of side effects is defined as follows:
| very common | occur in more than 1 in 10 patients |
| common | occur in 1 to 10 patients in 100 |
| uncommon | occur in 1 to 10 patients in 1,000 |
| rare | occur in 1 to 10 patients in 10,000 |
| very rare | occur in less than 1 patient in 10,000 |
| not known | frequency cannot be estimated from the available data |
In very rare cases, the medicine may cause severe allergic reactions.
The following symptoms may occur even after a single dose of Levomer eye drops:
- swelling and feeling of pressure in the throat,
- difficulty breathing. In rare cases, other allergic reactions may occur. Their symptoms include:
- increased redness and itching of the eyes,
- increased or sudden swelling of the eyelids. If any of these symptoms occur, discontinue the use of Levomer eye drops and contact your doctor immediately.
Common side effects (occur in 1 to 10 patients in 100):
- eye burning sensation,
- worsening of vision or mucous discharge in the eye.
Uncommon side effects (occur in 1 to 10 patients in 1,000):
- eye stinging or irritation,
- eye pain,
- eye dryness or discomfort,
- swelling or redness (inflamed eyes) of the conjunctiva (the front layer covering the eye) or eyelids,
- increased sensitivity to light,
- eye itching,
- eyelid stickiness,
- headache,
- rash around the eyes,
- stuffy nose or nasal discharge.
Rare side effects (occur in 1 to 10 patients in 10,000)
- allergic reactions, including skin rash.
Very rare side effects (occur in less than 1 patient in 10,000)
- swelling and feeling of pressure in the throat,
- difficulty breathing.
Additional side effects in children and adolescents
It is expected that the frequency, type, and severity of side effects in children and adolescents are the same as in adults.
If side effects occur, including any side effects not listed in this leaflet, tell your doctor or pharmacist.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will allow for more information to be collected on the safety of this medicine.
5. How to store Levomer eye drops, solution
Keep the medicine out of the sight and reach of children.
The bottle should be tightly closed.
Do not use this medicine after the expiry date stated on the bottle. The expiry date refers to the last day of the month.
Levomer eye drops are suitable for use for 28 days after first opening the bottle. After this time, the bottle with the remaining solution should be given to a pharmacist, who will dispose of it safely.
To prevent infections, after 28 days from the first opening, the currently used bottle should be discarded and a new one started.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Levomer eye drops, solution contain
- The active substance of the medicine is levofloxacin (in the form of hemihydrate) 5 mg/ml.
- The other ingredients are benzalkonium chloride, sodium chloride, sodium hydroxide (to adjust pH), hydrochloric acid (to adjust pH), and water for injections.
What Levomer eye drops, solution look like and contents of the pack
A clear yellowish-green aqueous solution.
5 ml solution in an LDPE bottle with a tamper-evident closure, with a dropper made of LDPE and a cap made of HDPE.
Marketing authorization holder:
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Manufacturer:
RAFARM S.A.
Thiesi Pousi-Xatzi
Agiou Louka
19002 Paiania, Attiki
Greece
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Portugal:
Levofloxacina Velka Hellas
Poland:
Levomer
Italy:
VISULFOX
United Kingdom:
Levofloxacin 5 mg/ml Eye Drops, Solution
Date of last revision of the leaflet:November 2020
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAdamed Pharma S.A. Rafarm S.A. Pharmaceutical Industry
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LevomerDosage form: Drops, 5 mg/mlActive substance: levofloxacinManufacturer: Rafarm S.A.Prescription not requiredDosage form: Drops, 5 mg/mlActive substance: levofloxacinPrescription requiredDosage form: Drops, 5 mg/mlActive substance: levofloxacinManufacturer: Unimed Pharma spol. s.r.o.Prescription required
Alternatives to Levomer in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Levomer in Україна
Alternative to Levomer in Іспанія
Online doctors for Levomer
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Levomer – subject to medical assessment and local rules.














