
Levetiracetam Eugia

Ask a doctor about a prescription for Levetiracetam Eugia

How to use Levetiracetam Eugia
Package Leaflet: Information for the User
Levetiracetam Eugia, 100 mg/mL, Concentrate for Solution for Infusion
Levetiracetam
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- -Keep this leaflet. You may need to read it again.
- -If you have any further questions, ask your doctor or pharmacist.
- -This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- -If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Pack
- 1. What Levetiracetam Eugia is and what it is used for
- 2. Before you take Levetiracetam Eugia
- 3. How to take Levetiracetam Eugia
- 4. Possible side effects
- 5. How to store Levetiracetam Eugia
- 6. Contents of the pack and other information
1.
What Levetiracetam Eugia is and what it is used for
Levetiracetam is an antiepileptic medicine (a medicine used to treat seizures in epilepsy).
Levetiracetam Eugia is used:
- as monotherapy (Levetiracetam Eugia used alone) in adults and adolescents from 16 years of age with newly diagnosed epilepsy, for the treatment of certain types of epilepsy. Epilepsy is a condition where patients have repeated seizures (fits). Levetiracetam is used for the treatment of epilepsy where seizures are initially limited to one part of the brain, but may spread to other parts of the brain (partial onset seizures with or without secondary generalisation). Your doctor has prescribed levetiracetam to reduce the number of seizures.
- as an adjunctive therapy (added on to other antiepileptic medicines):
- in adults, adolescents and children from 4 years of age, for the treatment of partial onset seizures with or without secondary generalisation,
- in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy, for the treatment of myoclonic seizures (short, shock-like jerks of a muscle or a group of muscles),
- in adults and adolescents from 12 years of age with idiopathic generalized epilepsy (a type of epilepsy that is thought to have a genetic cause), for the treatment of primary generalized tonic-clonic seizures (major fits, with loss of consciousness).
Levetiracetam Eugia, concentrate for solution for infusion is an alternative for patients when oral administration is temporarily not feasible.
2. Before you take Levetiracetam Eugia
Do not take Levetiracetam Eugia
- If you are allergic to levetiracetam, pyrrolidone derivatives or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before taking Levetiracetam Eugia, talk to your doctor:
- If you have kidney problems, Levetiracetam Eugia should be used with caution. Your doctor may need to adjust the dose.
- If you notice any slowing of growth or unexpected pubertal delay in your child, consult your doctor.
- Some patients with epilepsy treated with antiepileptic medicines such as Levetiracetam Eugia have had thoughts of harming or killing themselves. If at any time you have these thoughts, immediately contact your doctor.
- If you or your family/carer notice any change in your behaviour or if you have any unusual thoughts or if you develop suicidal thoughts and/or behaviour, you should immediately consult your doctor.
Should you experience any of the following, tell your doctor or pharmacist immediately:
- Abnormal thoughts, hostility or aggression, anxiety, depression, restlessness or mood swings, suicidal thoughts or behaviour.
- Worsening of seizures, especially at the beginning of the treatment or when the dose is increased.
If you experience any of these symptoms, contact your doctor immediately.
Children and adolescents
Levetiracetam Eugia should not be used in children and adolescents below 16 years of age for monotherapy (treatment with Levetiracetam Eugia alone).
Other medicines and Levetiracetam Eugia
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Levetiracetam Eugia may cause drowsiness. Therefore, you should not drive or use machines until you are sure that you are not affected.
Levetiracetam Eugia contains sodium
For doses of 250 mg and 500 mg:
This medicine contains less than 1 mmol (23 mg) of sodium per dose, i.e. it is essentially sodium-free.
For a dose of 1000 mg:
This medicine contains 38 mg of sodium (a major component of cooking salt) per dose, which is 1.9% of the maximum recommended daily intake of sodium for adults.
For a dose of 1500 mg:
This medicine contains 57 mg of sodium (a major component of cooking salt) per dose, which is 2.85% of the maximum recommended daily intake of sodium for adults.
3. How to take Levetiracetam Eugia
Levetiracetam Eugia is given as an intravenous infusion by a doctor or nurse.
Levetiracetam Eugia must be taken twice daily, in the morning and evening, at about the same time each day.
The intravenous formulation is an alternative for patients when oral administration is temporarily not feasible. Switching between the oral and intravenous formulation can be done without adjustment of the daily dose. The total daily dose and frequency of administration remain unchanged.
Adjunctive therapy and monotherapy (from 16 years of age)
Adults (from 18 years of age) and adolescents (from 12 to 17 years of age) with a body weight of 50 kg or more:
Recommended dose: 1000 mg to 3000 mg per day.
If you are taking Levetiracetam Eugia for the first time, your doctor will prescribe a lower dose for the first two weeks, then the lowest daily dose.
Dosing for children (from 4 to 11 years of age) and adolescents (from 12 to 17 years of age) with a body weight below 50 kg:
Recommended dose: 20 mg/kg body weight to 60 mg/kg body weight per day.
Method and route of administration:
Levetiracetam Eugia is administered intravenously.
The recommended dose should be diluted in at least 100 mL of a suitable diluent and administered as an infusion over more than 15 minutes.
More detailed information for doctors and nurses on the proper administration of Levetiracetam Eugia is provided in section 6.
Duration of treatment:
- There are limited data on the use of intravenous levetiracetam for a period longer than 4 days.
Withdrawal of Levetiracetam Eugia:
If treatment with Levetiracetam Eugia is to be discontinued, it should be gradually withdrawn to avoid an increase in seizure frequency. If your doctor decides to stop your treatment with Levetiracetam Eugia, he/she will tell you how to do it.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very serious side effects, requiring immediate medical attention
- -weakness, dizziness or difficulty breathing, as these may be signs of a serious allergic reaction (anaphylaxis)
- -swelling of the face, lips, tongue and throat (Quincke's oedema)
- -flu-like symptoms and rash on the face, then on the rest of the body, high temperature, increased liver enzymes in the blood, increased number of certain white blood cells (eosinophilia), swollen lymph nodes (Drug Reaction with Eosinophilia and Systemic Symptoms, DRESS)
- -symptoms such as decreased urine output, tiredness, nausea, vomiting, confusion and swelling of the feet, ankles or hands, as these may be signs of acute kidney injury;
- -skin rash which may lead to blistering and peeling of the skin (Stevens-Johnson syndrome, toxic epidermal necrolysis)
- -severe skin rash which may lead to skin peeling (Stevens-Johnson syndrome, toxic epidermal necrolysis)
- -signs of serious mental changes, or if you notice signs of confusion, somnolence, memory impairment (amnesia), abnormal behaviour, hallucinations, aggression, restlessness or manic behaviour, or if you experience suicidal thoughts or behaviour.
The most common side effects are: nasopharyngitis, somnolence, headache, fatigue and dizziness. At the beginning of the treatment or after an increase in the dose, side effects like somnolence, fatigue or dizziness may be more common. These effects should decrease over time.
Very common:may affect more than 1 in 10 people
- nasopharyngitis;
- somnolence, headache.
Common:may affect up to 1 in 10 people
- anorexia (loss of appetite);
- depression, hostility or aggression, anxiety, insomnia, nervousness or irritability;
- seizures, ataxia (impaired coordination), dizziness (unsteadiness), somnolence (drowsiness), tremor (shakiness);
- vertigo (dizziness);
- cough;
- abdominal pain, diarrhoea, dyspepsia (indigestion), vomiting, nausea;
- rash;
- asthenia/fatigue (tiredness).
Uncommon:may affect up to 1 in 100 people
- decreased platelet count, decreased white blood cell count;
- weight decrease, weight increase;
- suicidal ideation, mental disorders, abnormal behaviour, hallucinations, aggression, restlessness or manic behaviour, mood swings/emotional changes, panic attacks, emotional lability/mood changes, agitation;
- amnesia (memory loss), memory impairment (forgetfulness), ataxia (impaired coordination), paraesthesia (tingling), attention deficit/hyperactivity disorder;
- diplopia (double vision), blurred vision;
- abnormal liver function tests;
- alopecia (hair loss), rash, pruritus (itching);
- muscle weakness, muscle pain;
- injury.
Rare:may affect up to 1 in 1,000 people
- infection;
- decreased all blood cell counts;
- severe allergic reactions (DRESS, Quincke's oedema);
- hyponatraemia (low sodium levels in the blood);
- suicide, personality disorders (behavioural problems), thinking disorders (slow thinking, inability to concentrate);
- delirium;
- encephalopathy (a brain disease that can cause a variety of symptoms, including confusion, tremors and difficulty with speech and language);
- seizures may worsen or become more frequent;
- involuntary muscle contractions of the head, torso and limbs, difficulty in controlling movements, hyperkinesia (excessive restlessness);
- changes in heart rhythm (electrocardiogram);
- pancreatitis;
- liver dysfunction, hepatitis;
- sudden worsening of kidney function;
- rash on the skin which may lead to blistering and peeling of the skin (Stevens-Johnson syndrome, toxic epidermal necrolysis);
- rhabdomyolysis (breakdown of muscle tissue) and the associated increase in creatine phosphokinase in the blood. The incidence is significantly higher in Japanese patients compared to non-Japanese patients;
- stuttering or difficulty walking;
- occurring simultaneously: fever, muscle stiffness, unstable blood pressure and heart rate, confusion, decreased consciousness (these may be symptoms of a condition called neuroleptic malignant syndrome). The incidence is significantly higher in Japanese patients compared to non-Japanese patients.
Very rare:may affect up to 1 in 10,000 people
- repetitive, unwanted thoughts or a feeling of compulsion to perform a specific action (obsessive-compulsive disorder).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in the Medicines and Healthcare products Regulatory Agency (MHRA) Yellow Card Scheme (www.mhra.gov.uk/yellowcard). By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Levetiracetam Eugia
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial and carton after EXP. The expiry date refers to the last day of that month.
There are no special storage instructions for this medicine.
Chemical and physical stability during use has been demonstrated for 24 hours at 2-8°C and 15-25°C.
From a microbiological point of view, unless the method of opening/ dilution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
Do not use Levetiracetam Eugia if you notice any particles or discolouration.
6. Contents of the pack and other information
What Levetiracetam Eugia contains
- -The active substance is levetiracetam. Each mL of solution for infusion contains 100 mg of levetiracetam.
- The other ingredients are: sodium chloride, sodium acetate trihydrate, glacial acetic acid, water for injections.
What Levetiracetam Eugia looks like and contents of the pack
Levetiracetam Eugia, concentrate for solution for infusion is a clear, colourless solution.
Levetiracetam Eugia, concentrate for solution for infusion 5 mL is packaged in cartons of 10 vials.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Eugia Pharma (Malta) Limited
Vault 14, level 2
Valletta Waterfront
Floriana, FRN 1914
Malta
e-mail: [email protected]
Manufacturer/Importer:
APL Swift Services (Malta) Limited
HF26, Hal Far Industrial Estate, Hal Far
Birzebbugia, BBG 3000
Malta
This medicinal product is authorised in the Member States of the European Economic Area under the following names:
Belgium:
Levetiracetam AB 100 mg/ml, concentraat voor oplossing voor infusie
France:
LEVETIRACETAM ARROW LAB 100 mg/ml, solution à diluer pour perfusion
Italy:
Levetiracetam Aurobindo Italia 100 mg/ml concentrato per soluzione per infusione
Netherlands:
Levetiracetam Eugia 100 mg/ml, concentraat voor oplossing voor infusie
Portugal:
Levetiracetam Aurovitas 100 mg/ml concentrado para solução para perfusão
Spain:
Levetiracetam Aurovitas 100 mg/ml concentrado para solución para perfusión EFG
Poland:
Levetiracetam Eugia
Date of last revision of the leaflet: -----------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Instructions for proper use of Levetiracetam Eugia are provided in section 3.
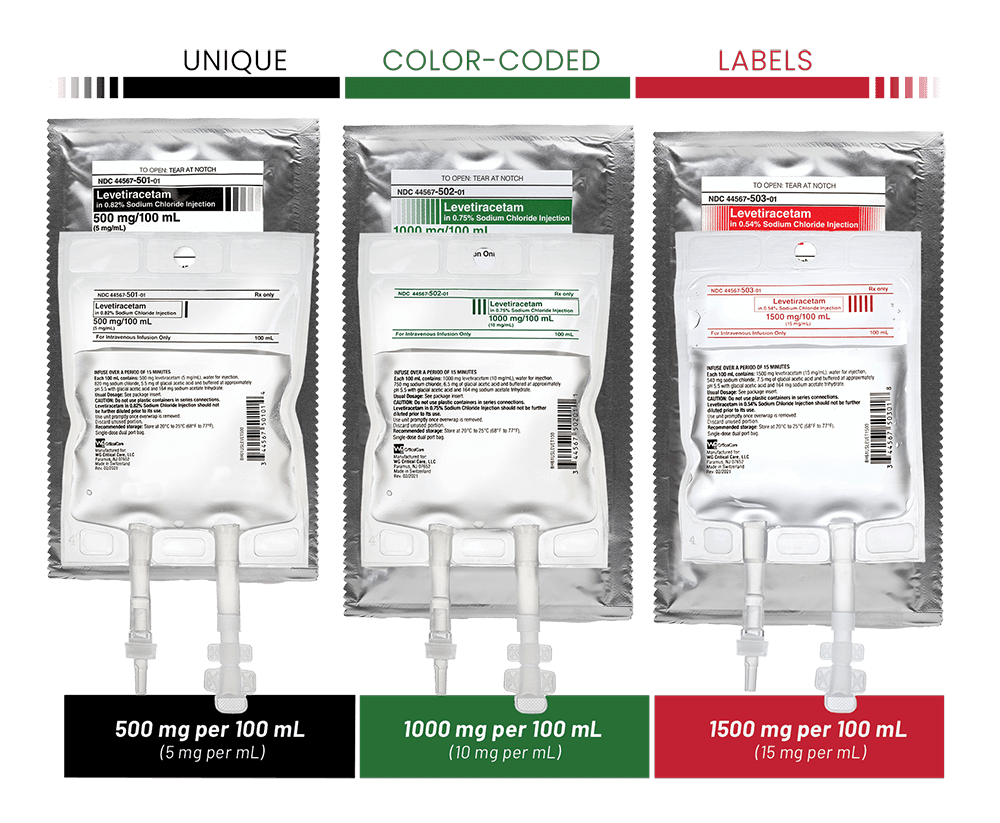
One vial of Levetiracetam Eugia concentrate contains 500 mg levetiracetam (5 mL concentrate 100 mg/mL). Table 1 provides recommendations for preparation and administration of Levetiracetam Eugia concentrate to achieve the total daily dose of 500 mg, 1000 mg, 2000 mg or 3000 mg divided into two doses.
Table 1. Preparation and administration of Levetiracetam Eugia concentrate
| Dose | Volume of Levetiracetam Eugia to be diluted | Volume of diluent | Infusion time | Frequency of administration | Total daily dose |
| 250 mg | 2.5 mL (half of a 5 mL vial) | 100 mL | 15 minutes | Twice daily | 500 mg/day |
| 500 mg | 5 mL (one 5 mL vial) | 100 mL | 15 minutes | Twice daily | 1000 mg/day |
| 1000 mg | 10 mL (two 5 mL vials) | 100 mL | 15 minutes | Twice daily | 2000 mg/day |
| 1500 mg | 15 mL (three 5 mL vials) | 100 mL | 15 minutes | Twice daily | 3000 mg/day |
This medicinal product is for single use only; any unused solution should be discarded.
Levetiracetam concentrate for solution for infusion is physically and chemically compatible with the following diluents for at least 24 hours at controlled room temperature (15-25°C) at concentrations from 2.5 mg/mL to 13 mg/mL.
- Sodium chloride 9 mg/mL (0.9%) solution for injection
- Ringer's solution for injection
- Dextrose 50 mg/mL (5%) solution for injection
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAPL Swift Services (Malta) Ltd.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Levetiracetam EugiaDosage form: Tablets, 250 mgActive substance: levetiracetamPrescription requiredDosage form: Tablets, 500 mgActive substance: levetiracetamPrescription requiredDosage form: Tablets, 750 mgActive substance: levetiracetamPrescription required
Alternatives to Levetiracetam Eugia in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Levetiracetam Eugia in Spain
Alternative to Levetiracetam Eugia in Ukraine
Online doctors for Levetiracetam Eugia
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Levetiracetam Eugia – subject to medical assessment and local rules.







