

Letibo

Ask a doctor about a prescription for Letibo

How to use Letibo
Package Leaflet: Information for the User
Letybo
Botulinum toxin type A
50 units, powder for solution for injection
Read all of this leaflet carefully before using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- 1. What Letybo is and what it is used for
- 2. Before you use Letybo
- 3. How to use Letybo
- 4. Possible side effects
- 5. How to store Letybo
- 6. Contents of the pack and other information
1. What Letybo is and what it is used for
Letybo contains the active substance botulinum toxin type A. Its action is to block nerve impulses to the muscles into which it is injected. This prevents muscle contraction, leading to a temporary paralysis of the muscle.
Letybo is used in adults under 75 years of age for the temporary improvement of moderate to severe vertical frown lines between the eyebrows, when the presence of these lines has a significant psychological impact on the patient.
2. Before you use Letybo
When not to use Letybo:
- if you are allergic to botulinum toxin type A or any of the other ingredients of this medicine (listed in section 6);
- if you have muscle disorders, such as myasthenia gravis, Lambert-Eaton syndrome, or amyotrophic lateral sclerosis;
- if you have an acute infection or inflammation at the planned injection sites.
Warnings and precautions
Before using Letybo, you should discuss with your doctor if you have:
- any disorder that affects the muscles or their nervous control;
- difficulty swallowing or breathing, now or in the past;
- bleeding disorders.
If you have a history of these disorders, Letybo is not recommended.
Pain associated with needle injection or fear of injections may cause fainting due to a sudden drop in blood pressure.
Very rarely, side effects have been reported in connection with the spread of botulinum toxin to distant sites from the injection site, such as excessive muscle weakness. Swallowing and breathing difficulties are serious reactions that can be life-threatening.
Patients or their caregivers should be instructed to seek medical attention immediately if they experience swallowing, speech, or breathing difficulties.
Children and adolescents
Letybo should not be used in children and adolescents under 18 years of age.
Letybo and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take.
Interactions may occur between Letybo and medicines:
- that interfere with nerve impulses to muscles;
- used to treat bacterial infections, such as spectinomycin or aminoglycoside antibiotics;
- that contain botulinum toxin available in other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Letybo should not be used during pregnancy or breastfeeding, or in women of childbearing potential not using effective contraception.
Driving and using machines
Botulinum toxin type A may cause weakness, dizziness, and vision disturbances. In such cases, the ability to react may be impaired, and you should not drive or operate machinery.
Letybo contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which is essentially sodium-free.
3. How to use Letybo
The units of botulinum toxin stated in this leaflet are specific to Letybo and cannot be compared to or converted into units of any other botulinum toxin product.
Letybo should only be administered by an appropriately qualified physician who has the necessary equipment and expertise to administer the product. A detailed description of the preparation of the solution and instructions for use are provided in the section "Information intended for healthcare professionals only" at the end of this leaflet.
Recommended dose
20 units divided into five injections of 0.1 mL (4 units). Each injection is administered into the muscle above or between the eyebrows.
Letybo is for intramuscular (i.m.) use only.
After preparation of the solution, the vial may only be used for a single session in a single patient. Unused solution should be discarded in accordance with the description found after section 6 in the information intended for healthcare professionals only.
A minimum of three months should be maintained between two treatment sessions with Letybo.
Overdose of Letybo
Overdose may cause muscle or nerve paralysis. Symptoms of overdose may appear immediately after injection.
In case of overdose, the doctor will monitor the patient for symptoms such as general weakness or muscle paralysis. Emergency services should be called immediately or the patient should go to the hospital if symptoms of botulinum toxin type A poisoning occur, such as:
- general weakness,
- drooping eyelid or double vision,
- swallowing and speech difficulties,
- partial paralysis of the respiratory muscles.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are mild to moderate, occur within the first few days after injection, and are temporary.
Some side effects can be serious. If you experience any of the following side effects, contact your doctor immediately or ask a relative to do so and go to the emergency department:
Rarely, may occur in less than 1 in 100 people
- drooping eyelid, eyelid spasms.
Very rarely, may occur in less than 1 in 1,000 people
- eye sensation disorders, eyebrow drooping,
- conjunctival hemorrhage,
- eye pain, dry eye, limited field of vision, blurred vision,
- throat sensation disorders,
- constipation,
- speech disorders.
Very rarely, may occur in less than 1 in 10,000 people
- muscle weakness,
- swallowing difficulties,
- inhalation of food or liquid into the airways or lungs,
- breathing difficulties.
In addition to these possible side effects, a severe allergic reaction may cause the following symptoms:
- swallowing, breathing, or speaking difficulties due to face, lip, tongue, or throat swelling; apart from these symptoms, hives may occur (see section 2).
Other known side effects may occur with the following frequency. Tell your doctor or pharmacist if they become severe:
Common, may occur in less than 1 in 10 people
- headache,
- injection site reactions.
Uncommon, may occur in less than 1 in 100 people
- head discomfort,
- local swelling, e.g., eyelid, face, or eye area,
- pain, bruising, swelling, itching, or redness at the injection site,
- bruising, e.g., around the eyes,
- infections, e.g., upper respiratory tract infections, such as the common cold,
- Mephisto effect (raising of the lateral eyebrows).
Rare, may occur in less than 1 in 1,000 people
- migraine,
- folliculitis,
- dizziness,
- abnormal sensations, e.g., tingling, pricking, or itching,
- nausea,
- dry skin, hives, itching,
- facial pain,
- fever,
- oral herpes,
- increased blood potassium levels,
- flu-like symptoms.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Side effects can be reported to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301; Fax: +48 22 49 21 309;
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Letybo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C – 8°C).
Reconstituted solution
It has been demonstrated that the ready-to-use product maintains its chemical and physical stability for 24 hours at 2°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, the responsibility for the storage conditions and expiration date of the reconstituted solution lies with the user, but the storage time should not exceed 24 hours at 2°C – 8°C, unless reconstitution/dilution is performed under controlled and validated aseptic conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Letybo contains
- The active substance is botulinum toxin type A.
- One vial contains 50 units of botulinum toxin type A produced by Clostridium botulinum.
- After preparation of the solution, each 0.1 mL dose contains 4 units.
- The other excipients are human albumin and sodium chloride.
What Letybo looks like and contents of the pack
Letybo is a white powder for solution for injection supplied in a 5 mL clear glass vial (Type I) with a rubber stopper and an aluminum cap, in a cardboard box.
A single pack contains one or two vials.
A multipack containing 2 cardboard boxes; each cardboard box contains one vial.
A multipack containing 6 cardboard boxes; each cardboard box contains one vial.
Not all pack sizes may be marketed.
Marketing authorization holder
Croma-Pharma GmbH
Industriezeile 6
2100 Leobendorf
Austria
Email: [email protected]
Phone: +43 2262 684 68-0
Manufacturer
Croma-Pharma GmbH
Cromazeile 2
2100 Leobendorf
Austria
Date of last revision of the leaflet: 07/2023
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
The units of botulinum toxin of this product cannot be compared to or converted into units of any other botulinum toxin product. The recommended doses expressed in units are different from those of other products containing botulinum toxin.
Instructions for use, preparation, and disposal of the medicinal product should be strictly followed.
Preparation of the solution
Reconstitution should be performed in accordance with good clinical practice, particularly using aseptic technique.
To reconstitute, 1.25 mL of sodium chloride injection solution at a concentration of 9 mg/mL (0.9%) is added to the Letybo vial.
Good practice is to prepare the solution and prepare the syringe over a paper towel covered with a film in case of product spillage. The 9 mg/mL (0.9%) sodium chloride injection solution should be drawn into a syringe and gently injected into the vial to avoid foaming/air bubbles and vigorous mixing of the solution, which could cause denaturation. The vial should be discarded if the solvent does not draw into the vial under vacuum. The reconstituted Letybo solution should be clear, colorless, and free of particulate matter. Before use, the vial should be inspected to ensure that the product does not contain particulate matter.
Letybo should not be used if the reconstituted solution is not clear or contains particulate matter.
Reconstituted solution
It has been demonstrated that the ready-to-use product maintains its chemical and physical stability for 24 hours at 2°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, the responsibility for the storage conditions and expiration date of the reconstituted solution lies with the user, but the storage time should not exceed 24 hours at 2°C – 8°C, unless reconstitution/dilution is performed under controlled and validated aseptic conditions.
Solutions for injection that have been stored for more than 24 hours should be discarded.
Instructions for use
Intramuscular injections should be performed with a sterile insulin or tuberculin syringe with a capacity of 1 mL and a graduation of 0.01 mL and a needle size of 30 to 31 G.
Draw up 0.5 mL of the reconstituted Letybo solution into a sterile syringe and remove any air bubbles from the syringe barrel. The needle used for reconstitution should be removed and replaced with a new one for administration of the product.
Caution should be exercised to avoid injecting the product into a blood vessel.
To prevent eyelid ptosis (blepharoptosis), injections should be avoided near the levator palpebrae superioris muscle, especially in patients with a larger brow depressor complex. When injecting into the corrugator muscle, the first injection should be performed directly above the medial edge of the eyebrow. The second injection should be performed approximately 1 cm above the supraorbital ridge (the bony ridge felt above the upper part of the eyebrow), where the midlines of the two eyebrows meet.
The injection site in the procerus muscle is located just above the midline of the nasal bridge, where the horizontal frown lines between the medial ends of the eyebrows are formed.
When injecting into the medial ends of the corrugator muscle and the midline of the eyebrows, the injection sites should be at least 1 cm away from the supraorbital ridge (the bony ridge felt above the upper part of the eyebrow).
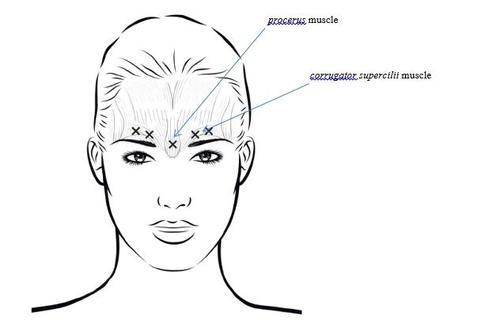
Injections should be performed carefully to avoid injecting the product into blood vessels. Before injection, the thumb or index finger can be placed below the orbital rim and pressed firmly to prevent the product from spreading into this area. The needle should be directed upwards and medially.
If the treatment fails after one month from the first treatment session, i.e., in case of no significant improvement compared to the initial state, the following steps can be considered:
- analysis of the reasons for the failure, i.e., injection into the wrong muscles, use of an inappropriate injection technique, formation of neutralizing antibodies, insufficient dose;
- re-evaluation of the justification for using botulinum toxin type A.
If no side effects occur after the previous treatment session, another treatment session can be performed provided that an interval of at least three months is maintained between them.
Procedure for safe disposal of used vials, syringes, and materials
To ensure safe disposal, the unused Letybo product should be reconstituted in the vial with a small amount of water and then sterilized in an autoclave. Empty vials, vials containing residual solution, syringes, and objects contaminated with the solution should be sterilized in an autoclave. Alternatively, any remaining Letybo product can be inactivated by adding a diluted solution of sodium hydroxide (0.1 N NaOH) or a diluted solution of sodium hypochlorite (0.5% or 1% NaOCl).
After inactivation, the used vials, syringes, and materials should not be emptied but should be placed in appropriate containers and disposed of in accordance with local regulations.
Recommendations for procedures in case of unforeseen events during administration of botulinum toxin
- Any spills should be cleaned up: using absorbent material soaked in a sodium hypochlorite solution (in the case of powder) or dry absorbent material in the case of the reconstituted product.
- Contaminated surfaces should be wiped with absorbent material soaked in a sodium hypochlorite solution and then dried.
- If the vial is broken, follow the above instructions. Carefully collect the broken glass fragments and wipe up any remaining product, avoiding cuts.
- If the product comes into contact with the skin, wash it with a sodium hypochlorite solution and then rinse thoroughly with water.
- In case of eye contact, rinse the eyes thoroughly with plenty of water or an eye wash solution.
- If the product gets into a wound, cut, or break in the skin, rinse the area thoroughly with plenty of water and take appropriate medical action depending on the dose to which the person was exposed.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterCroma-Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LetiboDosage form: Solution, 200 U Speywood/mlActive substance: botulinum toxinPrescription requiredDosage form: Powder, 125 Speywood unitsActive substance: botulinum toxinPrescription requiredDosage form: Powder, 125 Speywood unitsActive substance: botulinum toxinPrescription required
Alternatives to Letibo in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Letibo in Ukraine
Alternative to Letibo in Spain
Online doctors for Letibo
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Letibo – subject to medical assessment and local rules.














