

Lenzetto

Ask a doctor about a prescription for Lenzetto

How to use Lenzetto
Leaflet accompanying the packaging: patient information
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Lenzetto, 1.53 mg/dose, transdermal spray, solution
Estradiol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Lenzetto and what is it used for
- 2. Important information before using Lenzetto
- 3. How to use Lenzetto
- 4. Possible side effects
- 5. How to store Lenzetto
- 6. Contents of the packaging and other information
1. What is Lenzetto and what is it used for
Lenzetto is used in hormone replacement therapy (HRT). It contains the female hormone estrogen. Lenzetto is used in women after menopause, at least 6 months after the last natural menstrual period.
Lenzetto may also be used in women who have had their ovaries removed, as this causes immediate menopause.
Lenzetto is a spray that contains a small amount of the active substance called estradiol. After spraying directly onto the skin, the medicine passes through the skin into the bloodstream.
Lenzetto is indicated for use:
Relief of symptoms occurring after menopause
During menopause, the amount of estrogen produced by the woman's body decreases. This can cause symptoms such as a feeling of heat on the face, neck, and chest (so-called hot flashes). Lenzetto reduces the severity of these symptoms caused by menopause. The doctor will prescribe Lenzetto to the patient only if the symptoms are significantly bothering her daily life.
Lenzetto is indicated for the treatment of estrogen deficiency symptoms after menopause, when menstrual bleeding is no longer present. Estrogen deficiency symptoms include hot flashes (sudden surges of heat and sweating felt all over the body), sleep disturbances, irritability, and vaginal dryness.
Experience with the use of the medicine in women over 65 years of age is limited.
Lenzetto is not a contraceptive.
2. Important information before using Lenzetto
Medical history and regular check-ups
The use of Hormone Replacement Therapy (HRT) carries risks that need to be considered when deciding to start or continue treatment.
Experience with the use of the medicine in women with premature menopause (caused by ovarian dysfunction or surgery) is limited. The use of HRT in women with premature menopause may carry different risks than in women who have had a natural menopause. You should consult a doctor.
Before starting or restarting HRT, the doctor will take a medical history, including a family history. The doctor may decide to perform a physical examination. This may include a breast examination and, if necessary, a gynecological examination.
After starting Lenzetto, regular check-ups (at least once a year) should be performed, and the risk-benefit assessment of using Lenzetto should be discussed with the doctor.
According to the doctor's recommendations, regular breast examinations should be performed.
When not to use Lenzetto
If any of the following points apply to the patient, or if the patient has any doubts, they should consult a doctor before starting Lenzetto.
Lenzetto should not be used:
- if the patient has or has had breast cancer or is suspected of having it;
- if the patient has or is suspected of having other estrogen-dependent tumors, such as endometrial cancer (cancer of the lining of the uterus);
- if the patient has vaginal bleeding of unknown cause;
- if the patient has excessive thickening of the lining of the uterus (endometrial hyperplasia) that is not being treated;
- if the patient has or has had venous thromboembolic disease (blood clots in the legs or lungs);
- if the patient has disorders that affect blood clotting (such as protein C, protein S, or antithrombin deficiency);
- if the patient has or has recently had diseases caused by blood clots in the arteries, such as heart attack, stroke, or angina pectoris;
- if the patient has or has had liver disorders, and liver function tests have not returned to normal;
- if the patient has a rare blood disorder (porphyria) that runs in the family (it is a hereditary disease);
- if the patient is allergic to estradiol or any of the other ingredients of this medicine (listed in section 6).
If any of the above symptoms occur for the first time while using Lenzetto, treatment should be stopped immediately and a doctor consulted.
Warnings and precautions
Before starting Lenzetto, the patient should discuss it with their doctor or pharmacist.
If any of the following conditions are present, have been present in the past, or have worsened while using Lenzetto, the patient should inform their doctor, as they may require more frequent check-ups:
- uterine fibroids;
- increased risk of excessive thickening of the lining of the uterus (endometrial hyperplasia) and endometrial cancer;
- increased risk of blood clots;
- increased risk of estrogen-dependent tumors (such as breast cancer that occurred in the patient's mother, sister, or grandmother);
- high blood pressure;
- liver disorders, such as a benign liver tumor;
- diabetes;
- gallstones;
- migraine or severe headache;
- immune system disorders that affect multiple organs (systemic lupus erythematosus, SLE);
- epilepsy;
- asthma;
- disorders that affect the eardrum and hearing (otosclerosis);
- very high levels of fats in the blood (hypertriglyceridemia);
- fluid retention due to heart or kidney problems;
- hereditary and acquired angioedema.
Stop using Lenzetto and consult a doctor immediately
If the patient notices any of the following symptoms while using hormone replacement therapy (HRT):
- any of the conditions listed in the "When not to use Lenzetto" section;
- yellowing of the skin and eyes (jaundice), which may be signs of liver disorders;
- swelling of the face, tongue, and/or throat, and/or difficulty swallowing or hives, along with difficulty breathing, which may indicate angioedema;
- significant increase in blood pressure (symptoms may include headache, fatigue, dizziness);
- migraine headaches that occur for the first time;
- if the patient becomes pregnant;
- if the patient notices signs of a blood clot, such as:
- painful swelling and redness of the legs,
- sudden chest pain,
- difficulty breathing.
For more information, see the "Blood clots in the veins (thrombosis)" section.
Note:Lenzetto is not a contraceptive. If it has been less than 12 months since the last menstrual period, or if the patient is under 50 years old, it may be necessary to use a contraceptive method to protect against pregnancy.
The patient should consult a doctor.
HRT and cancer
Excessive thickening of the lining of the uterus (endometrial hyperplasia) and endometrial cancer.
The use of HRT containing only estrogen increases the risk of excessive thickening of the lining of the uterus (endometrial hyperplasia) and endometrial cancer.
This risk can be avoided by taking a progestogen in addition to estrogen for at least 12 days of each 28-day cycle. Therefore, women with a uterus will be prescribed a progestogen by their doctor. If the patient has had a hysterectomy (surgical removal of the uterus), they should consult their doctor to see if they can safely use this medicine without taking a progestogen.
Among women with a uterus who do not use HRT, endometrial cancer is diagnosed in about 5 out of 1000 women between the ages of 50 and 65.
Among women between the ages of 50 and 65 with a uterus who use HRT containing only estrogen, endometrial cancer is diagnosed in 10 to 60 out of 1000 women (i.e., 5 to 55 more cases), depending on the dose and duration of treatment.
Lenzetto contains a higher dose of estrogen than other medicines used for HRT that contain only estrogen. It is not known what the risk of endometrial cancer is when using Lenzetto with a progestogen.
Unexpected bleeding
While using Lenzetto, the patient will experience monthly bleeding (so-called withdrawal bleeding) if they are also taking progestogen sequentially. If the patient experiences unexpected bleeding or spotting that is not related to their monthly bleeding, which
- continues despite 6 months of treatment;
- appears for the first time after 6 months of using Lenzetto;
- continues despite stopping Lenzetto; the patient should see a doctor as soon as possible.
Breast cancer
Available data confirm an increased risk of breast cancer in women using hormone replacement therapy (HRT) in the form of combined estrogen and progestogen or estrogen-only therapy. This increased risk depends on the duration of HRT use. A higher risk is clearly visible after 3 years of HRT use. After stopping HRT, the additional risk will decrease over time, but the risk may persist for 10 years or more if HRT lasted more than 5 years.
Comparison
Among women between the ages of 50 and 54 who do not use HRT, breast cancer is diagnosed in about 13 to 17 out of 1000 women over 5 years.
In the case of 50-year-old women who start 5-year estrogen-only HRT, the number of cases will be 16 to 17 out of 1000 patients (i.e., 0 to 3 additional cases).
Among 50-year-old women who start 5-year combined estrogen and progestogen HRT, the number of cases will be 21 out of 1000 women (i.e., 4 to 8 more cases).
Among women between the ages of 50 and 59 who do not use HRT, breast cancer is diagnosed in about 27 out of 1000 women over 10 years.
In the case of 50-year-old women who start 10-year estrogen-only HRT, the number of cases will be 34 out of 1000 patients (i.e., 7 additional cases).
In the case of 50-year-old women who start 10-year combined estrogen and progestogen HRT, the number of cases will be 48 out of 1000 patients (i.e., 21 additional cases).
Regular breast examinations should be performed. If any of the following changes are found, the patient should consult a doctor:
- dimples in the breast skin,
- changes in the nipples,
- any visible or palpable lumps.
In addition, it is recommended that the patient participate in breast cancer screening programs if offered. It is essential to inform the nurse or person performing the mammogram about HRT use, as treatment may cause an increase in breast density, which can affect the mammogram results.
If breast density is increased, the mammogram may not detect all lumps.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of HRT containing only estrogen or combined estrogen and progestogen is associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer depends on age. For example, in women between the ages of 50 and 54 who do not use HRT, ovarian cancer is diagnosed in about 2 out of 2000 women over 5 years.
In women who have taken HRT for 5 years, it occurs in about 3 out of 2000 women (i.e., up to 1 additional case).
Effect of HRT on the heart and circulation
Blood clots in the veins (thrombosis)
The risk of blood clots in the veins is about 1.3 to 3 times higher in women using HRT than in women not using HRT, especially during the first year of treatment.
If a blood clot reaches the lungs, it can cause chest pain, shortness of breath, loss of consciousness, and even death.
The likelihood of blood clots in the veins increases with age and if any of the following points apply to the patient:
- if the patient is unable to walk for a long time due to a serious operation, injury, or illness (see section 3 "If the patient is to undergo surgery");
- if the patient is obese (BMI > 30 kg/m2);
- if the patient has a blood clotting disorder and needs long-term anticoagulant treatment;
- if any of the patient's close relatives have had a blood clot in their leg, lung, or other organ;
- if the patient has systemic lupus erythematosus (SLE);
- if the patient has cancer.
Symptoms of a blood clot include, see the "Stop using Lenzetto and consult a doctor immediately" section.
In a group of women over 50 years old who do not use HRT, blood clots in the veins are expected to occur in 4 to 7 out of 1000 women over 5 years.
Among women over 50 years old who have used combined estrogen and progestogen HRT for 5 years, there will be 9 to 12 cases out of 1000 women (i.e., 5 more cases).
Among women over 50 years old who have had a hysterectomy and have used estrogen-only HRT for 5 years, there will be 5 to 8 cases out of 1000 women (i.e., 1 more case).
Heart disease (heart attack)
There is no evidence that HRT prevents heart attacks.
In women over 60 years old who use combined estrogen and progestogen HRT, there is a slightly increased risk of heart disease compared to women not using HRT.
The risk of heart disease does not increase in women who have had a hysterectomy and use estrogen-only HRT.
Stroke
The risk of stroke is about 1.5 times higher in women using HRT than in women not using HRT. The increase in the number of stroke cases due to HRT use is proportional to the age of the population.
Comparison: In a group of women over 50 years old who do not use HRT, a stroke will occur in about 8 out of 1000 women over 5 years. Among women over 50 years old who use HRT, there will be about 11 stroke cases out of 1000 women over 5 years (i.e., 3 more cases).
Other conditions
HRT does not protect against memory loss. There is some evidence of an increased risk of memory loss in women who start HRT after the age of 65. The patient should consult a doctor.
Children
Estradiol in the form of a spray can be accidentally transferred from the patient's skin to other people.
Others, especially children, should not be allowed to come into contact with the exposed area of the patient's skin, and if necessary, the area should be covered with clothing after the spray has dried. If a child comes into contact with the area of skin where estradiol has been sprayed, they should wash the area of skin with soap and water as soon as possible. Due to the transfer of estradiol, small children may exhibit unexpected signs of sexual maturation (e.g., breast budding). In most cases, these symptoms will resolve when the child is no longer exposed to estradiol spray.
If a child who may have been accidentally exposed to estradiol spray shows any signs or symptoms of sexual maturation (breast development or other sexual changes), the patient should consult a doctor.
Lenzetto and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Some medicines may affect the action of Lenzetto. This may lead to irregular bleeding. These include:
- medicines used to treat epilepsy (such as phenobarbital, phenytoin, and carbamazepine),
- medicines used to treat tuberculosis (such as rifampicin, rifabutin),
- medicines used to treat HIV infection (such as nevirapine, efavirenz, ritonavir, and nelfinavir),
- herbal products containing St. John's Wort (Hypericum perforatum).
HRT may affect the action of other medicines:
- an antiepileptic medicine (lamotrigine), as it may increase the frequency of seizures,
- medicines used to treat hepatitis C virus (HCV) infection (such as a treatment regimen using ombitasvir/paritaprevir/ritonavir with or without dasabuvir, and a treatment regimen using glecaprevir/pibrentasvir) may cause an increase in liver enzyme levels in blood tests (increased ALT enzyme activity) in women using combined hormonal contraceptives containing ethinyl estradiol. Lenzetto contains estradiol instead of ethinyl estradiol. It is not known whether increased ALT enzyme activity may occur when using Lenzetto with this type of HCV treatment regimen.
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any over-the-counter medicines, herbal medicines, and natural products. The doctor will provide appropriate guidance.
Laboratory tests
If a blood test is necessary, the patient should inform their doctor or laboratory staff about Lenzetto use, as this medicine may affect the results of some tests.
Pregnancy and breastfeeding
Lenzetto is intended only for women after menopause. If the patient becomes pregnant, they should stop using Lenzetto immediately and consult a doctor.
Lenzetto should not be used during breastfeeding.
Driving and using machines
There is no data on the effect of Lenzetto on the ability to drive and use machines.
Lenzetto contains alcohol
This medicine contains 65.47 mg of alcohol (ethanol) per dose, which is equivalent to 72.74% v/v.
It may cause burning on damaged skin.
Alcohol-based liquids are flammable. Keep away from fire. When using the spray on the skin, avoid open flames, lit cigarettes, or certain hot devices (e.g., hair dryers) until the applied dose has dried.
3. How to use Lenzetto
This medicine should always be used as directed by the doctor. If the patient has any doubts, they should consult a doctor or pharmacist.
The doctor will try to prescribe the lowest possible dose for the shortest possible time to alleviate the patient's symptoms. During treatment, the doctor may adjust the dosage based on the patient's individual needs. If the patient thinks the effect of the medicine is too strong or too weak, they should consult a doctor.
If the patient has not had a hysterectomy (surgical removal of the uterus), the doctor will prescribe progestogen tablets to balance the effects of estrogen on the uterine lining. The doctor will explain how to take these tablets. At the end of the progestogen treatment, withdrawal bleeding may occur (see the "Unexpected bleeding" section).
If the patient is to undergo surgery
If the patient is planning to have surgery, they should tell their doctor about Lenzetto use. It may be necessary to stop using Lenzetto about 4 to 6 weeks before the operation to reduce the risk of blood clots (see section 2 "Blood clots in the veins"). The patient should ask their doctor when they can restart Lenzetto.
Where to apply Lenzetto
The spray should be applied to dry, healthy skin on the inner surface of the forearm. If this is not possible, the spray can be applied to the inner surface of the thigh.
Do not apply Lenzetto to the breasts or areas near them.
How to use Lenzetto
Before using a new applicator for the first time, prepare the pump by
spraying the medicine three times with the cap closed:Hold the container upright, as shown in Figure 1. Without removing the cap, press the thumb or index finger against the button three times.
The medicine is now ready to use.
The applicator should only be prepared before the first use of a new container, not before each use. If one or more doses are missed, the applicator should be prepared according to the instructions in the "Missed dose of Lenzetto" section.
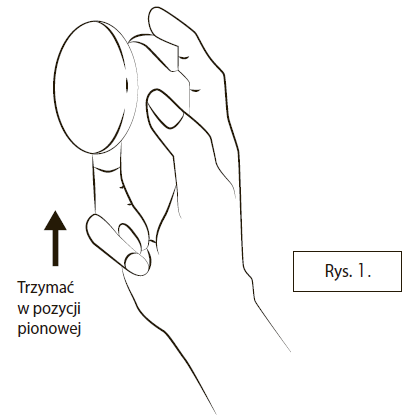
Make sure the skin where the medicine will be sprayed is healthy, clean, and dry.
Dosing instructions.
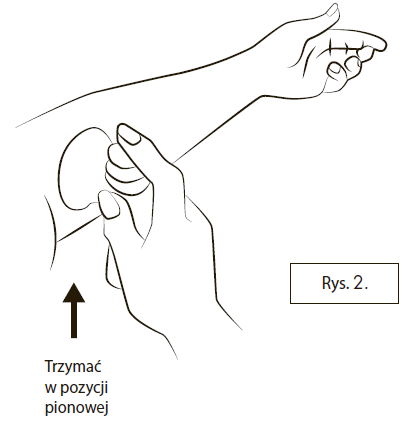
To administer the daily dose, remove the plastic cap, hold the container upright, and place the plastic applicator tip flat against the skin surface (Figure 2).
It may be necessary to move the arm or move the applicator outlet along the arm so that it fits snugly against the skin and there are no gaps between the applicator outlet and the skin.
Press the button once. Before releasing, press it all the way downand hold.
If it is necessary to spray again, move the applicator outlet along the arm so that it is outside the area where the medicine was previously sprayed. Press the button once.
If it is necessary to spray a third time, move the applicator outlet along the arm and press the button once.
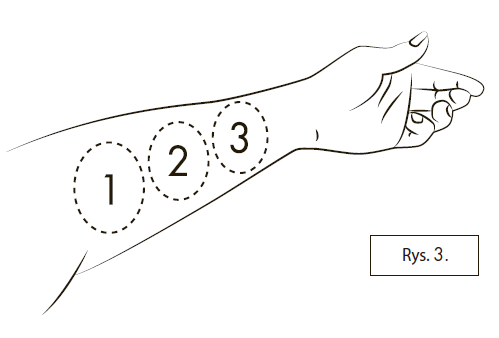
If it is not possible to perform the second or third spray on the same inner surface of the forearm, the medicine can be administered on the other forearm. If the patient has difficulty placing the applicator tip on the inner surface of the forearm, as shown in Figure 3, or has difficulty administering the medicine to the forearm, the medicine can also be administered to the inner surface of the thigh.
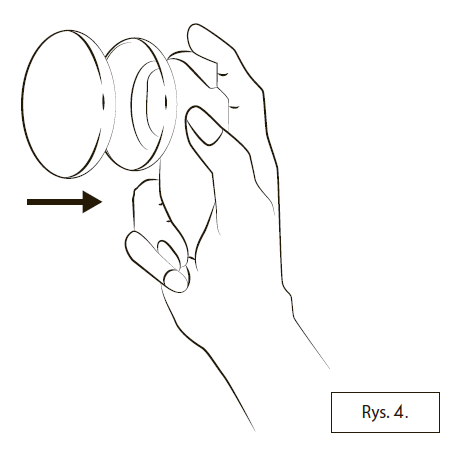
After using Lenzetto, the container should always be closed with the cap (Figure 4).
If the patient uses the medicine as directed, each spray will deliver the same amount of active substance to the skin, regardless of differences in the shape or appearance of the solution on the skin.
The sprayed medicine should be left to dry for at least 2minutes before dressing and at least 60minutes before bathing or showering. If Lenzetto gets on other areas of skin, such as the hands, the skin in that area should be washed with soap and water immediately.
Lenzetto should not be applied to damaged or injured skin.
Lenzetto should not be massaged or rubbed into the skin surface.
Do not allow others to touch the area of skin where the spray has been applied until it has dried and, if necessary, cover it with clothing 2 minutes after application.
If another person, especially a child, accidentally touches the skin surface where Lenzetto has been sprayed, they should wash the area of skin with soap and water.
What dose of Lenzetto to use
The doctor will likely recommend starting treatment with the lowest dose (one spray per day).
The patient should discuss with their doctor what dose of Lenzetto is suitable for them. If necessary, the doctor may increase the dose to two sprays per day. The maximum daily dose is 3 sprays.
How often to use Lenzetto
The total number of sprays (doses) prescribed by the doctor should be administered daily at the same time.
Duration of treatment with Lenzetto
Every 3-6 months, the patient should discuss with their doctor how long to use Lenzetto. Lenzetto should only be used for as long as symptoms related to menopause persist.
Other useful information
Sunscreen products may alter the absorption of estrogens in Lenzetto.
The patient should avoid using sunscreen products on the skin areas where they intend to spray Lenzetto. However, if sunscreen products need to be used, they should be applied to the skin at least 1 hour before using Lenzetto.
Lenzetto should be used with caution in extreme temperature conditions, such as saunas or sunbathing.
There is limited data suggesting that the rate and extent of Lenzetto absorption may be reduced in overweight and obese women. The patient should discuss this with their doctor. During treatment, the doctor may adjust the dose according to the patient's individual needs.
Using more Lenzetto than recommended
If more Lenzetto than recommended is used, or if the medicine is accidentally used by a child, the patient should consult a doctor or go to the hospital for advice on the risks and actions to take.
If more Lenzetto than recommended is used, nausea, vomiting, and irregular bleeding (unusual vaginal bleeding) may occur.
Missing a dose of Lenzetto
If a dose of Lenzetto is missed at the usual time, the patient should use the medicine as soon as possible, and then use it as usual. If it is almost time for the next dose, the patient should wait and use the next dose as usual. If one or more doses are missed, the applicator requires one preparatory spray with the cap closed. The patient should not use a double dose to make up for a missed dose. If a dose is missed, the likelihood of irregular bleeding and spotting may increase.
If the patient has any further doubts about using this medicine, they should consult a doctor or pharmacist.
Stopping Lenzetto
The doctor will also explain how to stop using the medicine after treatment is completed.
If the patient has any further doubts about using this medicine, they should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Lenzetto can cause side effects, although not everybody gets them.
Women using HRT are more likely to report the following conditions than women not using HRT:
- breast cancer;
- abnormal growth or cancer of the lining of the uterus (endometrial hyperplasia or cancer);
- ovarian cancer;
- blood clots in the veins of the legs or lungs (venous thromboembolism);
- heart disease;
- stroke;
- gallbladder disease;
- high blood pressure;
- liver disorders;
- high blood sugar levels;
- possible memory loss, if HRT is started after the age of 65.
For more information on the above side effects, see section 2.
Some side effects may be serious.
The following symptoms require immediate medical attention:
- sudden chest pain;
- chest pain that spreads to the arm or neck;
- difficulty breathing;
- painful swelling and redness of the legs;
- yellowing of the eyes and face (jaundice);
- unexpected vaginal bleeding (irregular bleeding) or spotting after using Lenzetto for some time or after stopping treatment;
- changes in the breasts, including dimpling of the breast skin, changes in the nipples, or any lumps that can be seen or felt;
- painful periods;
- dizziness and fainting;
- changes in speech;
- changes in vision;
- unexplained migraine-like headaches.
If any side effect worsens or if any side effects not listed in the leaflet occur, the patient should tell their doctor or pharmacist.
After using Lenzetto, the following side effects have been reported:
Common side effects(may affect up to 1 in 10 people)
Headache, abdominal pain, nausea, rash, itching (pruritus), irregular uterine bleeding or vaginal bleeding, including spotting, breast tenderness, breast pain, weight gain or weight loss.
Uncommon side effects(may affect up to 1 in 100 people)
Allergic reactions, depressed mood, insomnia (difficulty sleeping), dizziness (feeling of dizziness or "spinning"), vision disturbances, palpitations (feeling of heartbeat), diarrhea, dyspepsia (indigestion), high blood pressure, erythema multiforme (a condition characterized by painful red lumps on the skin), hives (generalized or localized rash or lumps), skin irritation, fluid retention (edema), muscle pain, breast discoloration, nipple discharge, uterine polyps or cervical polyps, endometrial hyperplasia, ovarian cyst, vaginal infection (vaginitis), increased liver enzyme activity and cholesterol levels in the blood, axillary pain.
Rare side effects(may affect up to 1 in 1000 people)
Anxiety, decreased or increased sexual desire, migraines, intolerance to contact lenses, bloating, vomiting, excessive hair growth on the body, acne, muscle cramps, painful periods, premenstrual syndrome, breast enlargement, fatigue.
After Lenzetto has been marketed, the following other side effectshave been reported, with a frequency of "unknown" (frequency cannot be estimated from available data): hair loss (alopecia), chloasma (golden-brown skin discoloration, so-called "pregnancy spots" especially on the face), skin discoloration.
The following side effects have been reported after using other HRT medicines:
Severe allergic reaction, which causes swelling of the face or throat (angioedema), anaphylactic and/or anaphylactoid reactions (severe allergic reaction that causes difficulty breathing or dizziness), glucose intolerance, depression, mood disorders, irritability, worsening of chorea (a condition characterized by involuntary movements), worsening of epilepsy, dementia, worsening of asthma, gallbladder disease, jaundice, pancreatitis, benign tumor of the smooth muscle of the uterus, skin disorders, skin discoloration, especially on the face or neck, known as "pregnancy spots" (chloasma), painful red lumps on the skin (erythema multiforme); rash in the shape of target-like lesions or ulcers (erythema multiforme), bleeding disorders (purpura), hair loss, joint pain, nipple discharge, breast lumps, increased size of benign tumors of the smooth muscle of the uterus, changes in the shedding of the inner lining of the cervix, vaginal infection (vaginitis), fungal infection of the vagina (vaginal thrush), low calcium levels in the blood.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Adverse Reaction Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Lenzetto
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
The medicine should be used within 56 days of first use.
Do not store in the refrigerator or freeze.
Do not store above 25°C.
The medicine contains ethanol, which is a flammable liquid. Keep away from heaters, open flames, and other ignition sources.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Lenzetto contains
- The active substance is estradiol (in the form of estradiol hemihydrate). Each dose contains 1.53 mg of estradiol (equivalent to 1.58 mg of estradiol hemihydrate).
- The other ingredients are octyl salicylate and ethanol 96%.
What Lenzetto looks like and contents of the pack
Lenzetto is a transdermal spray solution containing estradiol and octyl salicylate in ethanol. It is provided with a dosing pump.
Lenzetto is available in a glass bottle with a dosing pump, closed in a plastic applicator and protective cap, in a cardboard box. Each pack contains one bottle, containing 6.5 ml of solution, and allows for 56 doses of 90 microliters each.
The patient should mark the number of sprays used in the table on the packaging.
Each dose contains 1.53 mg of estradiol.
The Lenzetto container should not be used for more than the labeled number of sprays, even if the container is not completely empty.
Pack size:
One plastic container, 6.5 ml (56 doses).
For more detailed information, the patient should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Romania, the country of export:
Gedeon Richter România S.A.
Str. Cuza Vodă Nr. 99-105
540306 Târgu-Mureş, Romania
Manufacturer:
Gedeon Richter România S.A.
Str. Cuza Vodă Nr. 99-105
540306 Târgu-Mureş, Romania
Gedeon Richter Plc.
Gyömrői út 19-21
1103 Budapest, Hungary
Parallel importer:
InPharm Sp. z o.o., ul. Strumykowa 28/11, 03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k., ul. Chełmżyńska 249, 04-458 Warsaw
Authorization number in Romania, the country of export:13913/2021/01
13913/2021/02
Parallel import authorization number: 149/25
Date of leaflet approval: 24.04.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Gedeon Richter România S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LenzettoDosage form: Gel, 0.5 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mg/gActive substance: estradiolPrescription required
Alternatives to Lenzetto in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lenzetto in España
Alternative to Lenzetto in Ucrania
Online doctors for Lenzetto
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lenzetto – subject to medical assessment and local rules.










