
Itulazax

Ask a doctor about a prescription for Itulazax

How to use Itulazax
Package Leaflet: Information for the User
ITULAZAX
12 SQ-Bet, sublingual lyophilisate
For use in adults and children (aged 5 years and above)
Standardized allergen extract of white birch pollen (Betulaverrucosa)
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this package leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is ITULAZAX and what is it used for
- 2. Important information before taking ITULAZAX
- 3. How to take ITULAZAX
- 4. Possible side effects
- 5. How to store ITULAZAX
- 6. Contents of the pack and other information
1. What is ITULAZAX and what is it used for
What is ITULAZAX
ITULAZAX contains an allergen extract of birch pollen. The medicine is in the form of a sublingual lyophilisate, similar to a tablet, but softer and absorbed into the body after being placed under the tongue.
What is ITULAZAX used for
Treatment:
- allergic rhinitis and (or)
- conjunctivitis caused by pollen from trees such as birch, alder, hazel, oak, beech, and chestnut.
- Allergic rhinitis is an inflammatory condition of the nasal mucosa - causing sneezing, nasal congestion, or runny nose.
- Conjunctivitis is an inflammatory condition of the eyes - causing redness, itching, or tearing.
ITULAZAX is used in adults and children (aged 5 years and above). ITULAZAX is prescribed by doctors with experience in treating allergies.
How ITULAZAX works
ITULAZAX increases immunological tolerance (the body's ability to cope) to tree pollen.
How the doctor will decide if ITULAZAX is suitable for the patient
The doctor will assess the patient's allergic symptoms and perform skin tests and (or) order a blood test.
2. Important information before taking ITULAZAX
When not to take ITULAZAX:
- If the patient is allergic to any of the other ingredients of this medicine (listed in section 6);
- If lung function is poor - the doctor will decide;
- If the patient has had severe asthma or uncontrolled asthma attacks in the last three months - the doctor will decide;
- If the patient has a disease affecting the immune system, is taking immunosuppressive drugs, or has cancer;
- If the patient has recently had a tooth extraction, other oral surgery, has current mouth ulcers, or has lost a tooth. The doctor may recommend delaying the start of treatment or interrupting treatment until the oral wounds have healed.
Warnings and precautions
Before starting ITULAZAX, discuss with your doctor if:
- The patient is being treated for depression with tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), or is being treated for Parkinson's disease with catechol-O-methyltransferase (COMT) inhibitors;
- The patient has heart disease and (or) is being treated with beta-adrenergic blockers;
- The patient has had a severe allergic reaction to an injection containing a tree pollen allergen extract in the past;
- The patient has asthma and a current respiratory tract infection, such as a cold, sore throat, or pneumonia, on the day of the first dose of ITULAZAX. The doctor will delay the start of treatment until the patient feels better;
- The patient has had worsening of severe asthma in the last 12 months;
- The patient has a disease affecting the immune system or is taking immunosuppressive drugs;
- The patient is to receive a protective vaccination. The doctor will decide whether the patient can be vaccinated without interrupting ITULAZAX treatment;
- The patient has a fish allergy. ITULAZAX may contain trace amounts of fish protein. Available data do not indicate an increased risk of an allergic reaction in patients with a fish allergy.
If any of the above situations apply to the patient, they should talk to their doctor before taking ITULAZAX. If the patient experiences severe or persistent heartburn or difficulty swallowing, they should stop taking ITULAZAX and contact their doctor, as these may be symptoms of allergic esophagitis. ITULAZAX contains the allergen to which the patient is allergic, so mild to moderate allergic reactions can be expected. These reactions may occur in the mouth and throat. If the symptoms are bothersome, the patient should tell their doctor, who will decide whether the patient needs antihistamines. In the first few days of treatment at home, new allergic reactions may occur that were not observed on the first day of treatment in the doctor's office. Information on possible side effects can be found in section 4.
Children
ITULAZAX is not intended for use in children under 5 years of age.
ITULAZAX and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking, has recently taken, or might take, including those obtained without a prescription. If the patient is taking other anti-allergic medicines, such as antihistamines or corticosteroids, they should tell their doctor, who should assess the use of these medicines. If the patient stops taking anti-allergic medicines, they may experience more side effects of ITULAZAX.
ITULAZAX with food and drink
Do not eat or drink for 5 minutes after taking this medicine.
Pregnancy, breastfeeding, and fertility
There is no experience with the use of ITULAZAX during pregnancy. Do not start treatment with ITULAZAX during pregnancy. If the patient becomes pregnant during treatment, they should talk to their doctor about whether they can continue treatment. There is no experience with the use of ITULAZAX during breastfeeding. However, it is not expected to affect the breastfed child. Talk to your doctor about whether you can continue taking ITULAZAX while breastfeeding. There is no experience with the use of ITULAZAX in women planning to become pregnant. Talk to your doctor before taking this medicine if you are planning to become pregnant.
Driving and using machines
ITULAZAX has no or negligible influence on the ability to drive and use machines. However, the patient should assess whether the medicine affects their well-being and, in case of doubt, talk to their doctor or pharmacist.
3. How to take ITULAZAX
Always take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
How much ITULAZAX to take
- The recommended dose is usually one sublingual lyophilisate (soft tablet) per day.
How to take ITULAZAX
Start taking ITULAZAX at least 4 months before the expected start of the tree pollen season. The doctor will decide how long to take ITULAZAX. According to the guidelines, changes in the course of the underlying allergic disease may occur after 3 years of treatment with ITULAZAX. The efficacy of long-term treatment has not been established. If the symptoms of allergy do not improve during the first year of treatment with ITULAZAX, the patient should discuss with their doctor whether to continue treatment. The first dose of ITULAZAX should be taken in the doctor's office, because:
- After taking the first dose, the patient should be under medical supervision for at least half an hour.
- This precaution allows for the assessment of the patient's individual sensitivity to treatment.
- It also allows for discussion with the doctor about possible side effects.
Take ITULAZAX every day, even if it takes some time before an improvement occurs. Before touching the medicine, make sure your hands are dry.
The medicine should be taken as follows:
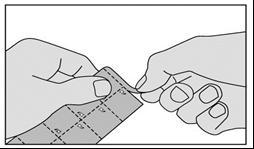
- 1. Tear off the strip marked with triangles at the top of the packaging.
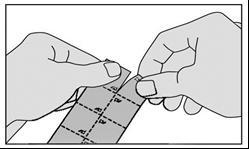
- 2. Tear off one square of the sublingual lyophilisate from the packaging along the perforation line.
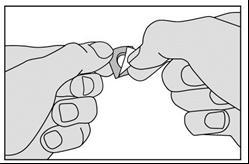
- 3. Bend the marked corner of the foil and then pull it.
- Do not push the medicine through the foil, as it can break easily.
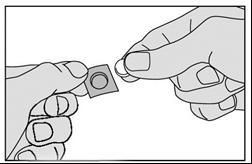
- 4. Carefully remove the medicine from the foil and take it immediately.
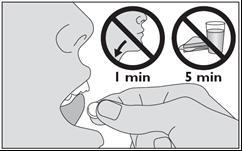
- 5. Place the medicine under your tongue. It should stay there until it dissolves.
- Do not swallow saliva for the first minute.
- Do not eat or drink for at least 5 minutes.
Use in children
ITULAZAX is not intended for use in children under 5 years of age.
Use in the elderly
Experience in the elderly (aged 65 years and above) is limited.
Taking a higher dose of ITULAZAX than recommended
If a higher dose of ITULAZAX than recommended is taken, the patient may experience unwanted allergic symptoms, including local symptoms in the mouth and throat. If the symptoms are severe, contact a doctor or go to the hospital immediately. See section 4.
Missing a dose of ITULAZAX
- If a dose is missed, take ITULAZAX later that day.
- Do not take a double dose to make up for a missed dose.
- If the patient forgets to take ITULAZAX for more than 7 days, they should contact their doctor before taking it again.
Stopping ITULAZAX treatment
The effectiveness of the treatment depends on taking the medicine as recommended by the doctor. If the patient has any further questions about the use of this medicine, they should ask their doctor or pharmacist.
4. Possible side effects
Like all medicines, ITULAZAX can cause side effects, although not everybody gets them. A side effect may be an allergic reaction to the allergen (pollen) that the patient is being treated for.
- In most cases, side effects are mild to moderate and occur within the first few days of treatment.
- Most of them should disappear within a few months of treatment, in most cases within one or two weeks.
If any side effect bothers the patient or is troublesome, they should contact their doctor, who will decide whether it is necessary to administer antihistamines to alleviate the symptoms. Side effects usually occur within 10 minutes of taking ITULAZAX, every day while taking the medicine, and decrease within an hour.
Severe side effects:
Frequency not known (cannot be estimated from the available data)
- Severe allergic/anaphylactic reaction
Stop taking ITULAZAX and immediately inform your doctor or go to the hospital if you experience any of the following symptoms:
- Symptoms of a severe allergic reaction:
- Significant worsening of existing asthma
- Severe throat swelling
- Difficulty swallowing
- Difficulty breathing
- Voice changes (e.g., hoarseness)
- Low blood pressure (hypotension)
- Feeling of fullness in the throat
Other possible side effects:
Very common (may affect more than 1 in 10 people):
- Itching of the ears, mouth, or tongue
- Swelling in the mouth
- Irritation of the throat
- Prickling sensation in the mouth
Common (may affect up to 1 in 10 people):
- Rhinitis
- Oral allergy syndrome (itching and (or) swelling of the mouth and throat after eating certain raw vegetables, fruits, or nuts)
- Changes in taste
- Eye symptoms (e.g., itching, tearing, swelling, redness)
- Cough
- Dry throat
- Hoarseness
- Shortness of breath
- Pain in the mouth or throat
- Throat swelling
- Abdominal pain
- Diarrhea
- Heartburn
- Pain when swallowing or difficulty swallowing
- Pain or burning sensation of the tongue
- Numbness in the mouth
- Swelling of the lips or tongue
- Itching of the lips
- Nausea
- Discomfort in the mouth
- Blisters in the mouth
- Prickling sensation in the throat
- Stomatitis
- Urticaria
- Discomfort in the chest
- Feeling of a foreign body in the throat
Uncommon (may affect up to 1 in 100 people):
- Feeling of tightness in the throat
- Glossitis
- Formation of blisters on the lips
- Oral ulcers
- Irritation of the esophagus
- Sudden swelling of the face, mouth, or throat
Frequency not known (cannot be estimated from the available data):
- Allergic esophagitis (eosinophilic esophagitis) If any side effect bothers the patient or is troublesome, they should contact their doctor, who will decide whether it is necessary to administer antihistamines to alleviate the symptoms.
Additional side effects in children
Side effects in children (aged 5 years and above) are similar to those in adults. Additionally, some side effects in children include: Common (may affect up to 1 in 10 people): redness in the mouth, discomfort in the tongue or throat, rash, urticaria, headache, and itching of the nose. Uncommon (may affect up to 1 in 100 people): anaphylactic reaction, rhinitis, pain in the esophagus, and excessive salivation.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Ministry of Health, or to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store ITULAZAX
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the blister and carton after: "Expiry date (EXP)". The expiry date refers to the last day of the month. Store in the original blister to protect from moisture. This medicine does not require any special storage conditions. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What ITULAZAX contains
The active substance of ITULAZAX is a standardized allergen extract of white birch pollen (Betulaverrucosa). The activity of one sublingual lyophilisate is expressed in SQ-Bet units. The activity of one sublingual lyophilisate is 12 SQ-Bet. One sublingual lyophilisate contains 194 micrograms of the Bet v 1 allergen. The other ingredients are: gelatin (derived from fish), mannitol, and sodium hydroxide (to adjust the pH).
What ITULAZAX looks like and contents of the pack
White or almost white, round sublingual lyophilisate with an embossed image on one side. Aluminum blisters with a removable aluminum foil in a cardboard box. Each blister contains 10 sublingual lyophilisates. The following pack sizes are available: 30 or 90 sublingual lyophilisates.
Marketing authorization holder
ALK-Abelló A/S Bøge Allé 6-8 DK-2970 Hørsholm Denmark
Manufacturer
ALK-Abelló S.A. Miguel Fleta 19 28037 Madrid Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Croatia, Czech Republic, Denmark, Finland, France, Germany, Ireland, Luxembourg, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, and Sweden: ITULAZAX Date of last revision of the package leaflet:04/2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterALK-Abello A/S
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ItulazaxDosage form: Solution, concentration "0" - 1 JS/ml, concentration "1" - 10 JS/ml, concentration "2" - 100 JS/ml, concentration "3" - 1000 JS/ml, concentration "4" - 5000 JS/ml (primary treatment); concentration "4" - 5000 JS/ml (maintenance treatment)Active substance: tree pollenPrescription not requiredDosage form: Suspension, 20,000 AU/mlActive substance: tree pollenManufacturer: Hal Allergy B.V.Prescription not requiredDosage form: Lyophilizate, 12 SQ-HDMActive substance: house dust mitesManufacturer: ALK-Abello S.A.Prescription required
Alternatives to Itulazax in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Itulazax in Ukraine
Alternative to Itulazax in Spain
Online doctors for Itulazax
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Itulazax – subject to medical assessment and local rules.














