
Infanrix-ipv

Ask a doctor about a prescription for Infanrix-ipv

How to use Infanrix-ipv
Leaflet attached to the packaging: Information for the user
Infanrix-IPV, suspension for injection in a pre-filled syringe
Vaccine against diphtheria, tetanus, pertussis (acellular, composite) and poliomyelitis (inactivated), adsorbed
Please read carefully the contents of the leaflet before using the vaccine, as it contains important information for the patient.
- Please keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This vaccine has been prescribed to a specific person. It should not be given to others.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Infanrix-IPV vaccine and what is it used for
- 2. Important information before using Infanrix-IPV vaccine
- 3. How to use Infanrix-IPV vaccine
- 4. Possible side effects
- 5. How to store Infanrix-IPV vaccine
- 6. Contents of the packaging and other information
1. What is Infanrix-IPV vaccine and what is it used for
Infanrix-IPV vaccine is used for booster and reminder vaccination in children to prevent four diseases:
- Diphtheria -a severe bacterial disease that most often affects the respiratory tract and sometimes the skin. In the respiratory tract, it leads to swelling, which causes serious breathing difficulties, and sometimes suffocation. Diphtheria bacteria also produce toxins that can cause nerve damage, heart disease, and even death.
- Tetanus -tetanus bacteria enter the human body through a cut, scratch, or wound in the skin. Injuries that pose the greatest risk of tetanus infection include: burns, fractures, deep wounds, or wounds contaminated with soil, dust, horse manure, or splinters. These bacteria produce toxins that can cause muscle stiffness, painful muscle spasms, seizures, and even death. Muscle spasms can be so strong that they lead to spinal fractures.
- Pertussis (whooping cough) -a highly contagious disease that affects the respiratory tract. It causes severe coughing attacks, which can lead to breathing problems. The cough in this disease is very characteristic - people with pertussis are said to "cough violently". The cough can last for 1-2 months or longer. Pertussis bacteria can also cause ear infections, bronchitis, which can last a long time, pneumonia, seizures, brain damage, and even death.
- Poliomyelitis (Polio) -is a viral infection. Polio often has a mild course. However, in some people, it can have a severe course and cause permanent damage, and even death. It can cause muscle paralysis (muscles cannot perform their functions). It can affect respiratory muscles or those that enable independent movement. Affected limbs can be deformed and painful.
Infanrix-IPV vaccine is intended for children from 16 months to 13 years old, inclusive. It is not intended for people over 14 years old.
How Infanrix-IPV vaccine works:
- The vaccine causes the body to produce its own immunity (antibodies). They will protect the vaccinated person against the mentioned diseases.
- The vaccine does not cause the diseases it protects against.
2. Important information before using Infanrix-IPV vaccine
When not to use Infanrix-IPV vaccine
- if the child is allergic to any of the components of Infanrix-IPV vaccine (components listed in section 6)
- or neomycin, polymyxin (antibiotics)
- or formaldehyde. Allergy symptoms may include an itchy skin rash, difficulty breathing, swelling of the face or tongue.
- if an allergic reaction to any diphtheria, tetanus, pertussis, or polio vaccine has occurred
- if a neurological disorder (encephalopathy) has occurred within 7 days of previous administration of a pertussis vaccine
- if the child has a high fever (above 38.0°C). A mild infection, such as a cold, should not be a contraindication to vaccination, but the doctor should be informed first.
In the above cases, Infanrix-IPV vaccine should not be administered. In case of doubts, the doctor or pharmacist should be consulted before the child receives Infanrix-IPV vaccine.
Warnings and precautions
Before the child receives Infanrix-IPV vaccine, the doctor or pharmacist should be informed:
- if the child has ever had health problems after vaccination with Infanrix-IPV or another pertussis vaccine, particularly:
- high fever (above 40°C) within 48 hours of vaccination
- collapse or shock-like state within 48 hours of vaccination
- persistent crying lasting at least three hours within 48 hours of vaccination
- seizures or febrile seizures within 3 days of vaccination
- if the child has an undiagnosed or progressive brain disease or untreated epilepsy. The vaccine should be administered after the disease has been controlled
- if the child has bleeding or bruising
- if the child has a history of febrile seizures or a family history of such seizures
- if the child has immune system disorders (including HIV infection). In such cases, Infanrix-IPV vaccine may be administered, but vaccinated patients may not develop adequate protection against infections.
After or even before each injection, fainting (especially in adolescents) may occur. Therefore, the doctor or nurse should be informed if the patient has ever fainted during an injection. In the above cases or in case of doubts, the doctor or pharmacist should be consulted before the child receives Infanrix-IPV vaccine.
Infanrix-IPV vaccine and other medicines
The doctor or pharmacist should be informed about all medicines the child is taking or has recently taken, as well as any medicines planned to be administered to the child. In particular, the doctor or pharmacist should be informed if the child is taking:
- medicines that affect the immune system or has undergone radiation therapy. In such cases, the vaccine may be administered, but it may not work as well. If possible, the vaccine should be administered after the treatment has been completed.
- other vaccines: Infanrix-IPV can be administered simultaneously with other vaccines. However, they should be administered at different sites on the body.
Pregnancy and breastfeeding
Administration of Infanrix-IPV vaccine to pregnant or breastfeeding women is very unlikely. This is because the vaccine is intended for use in children from 16 months to 13 years old (inclusive). The vaccine is not recommended during pregnancy or breastfeeding. The doctor should be consulted before taking any medicines.
Driving and using machines
Administration of Infanrix-IPV vaccine to individuals who will drive vehicles and operate machines is very unlikely. This is because the vaccine is intended for use in children from 16 months to 13 years old (inclusive). After vaccination, the child may feel sleepy. If this happens, the child should not drive vehicles, ride a bicycle, or operate machines and tools.
Infanrix-IPV vaccine contains neomycin, polymyxin (antibiotics), para-aminobenzoic acid, phenylalanine, sodium, potassium, and formaldehyde.
The vaccine should not be taken in case of allergy to any of these components. The doctor should be informed if the child has ever had an allergic reaction to these components. Infanrix-IPV contains para-aminobenzoic acid, which can cause allergic reactions (possible late reactions) and, exceptionally, bronchospasm. The vaccine contains 0.036 micrograms of phenylalanine per dose. Phenylalanine can be harmful to patients with phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates in the body because the body does not eliminate it properly. This vaccine contains less than 1 mmol (23 mg) of sodium per dose, which means the vaccine is considered "sodium-free". This vaccine contains potassium, less than 1 mmol (39 mg) of potassium per dose, which means the vaccine is considered "potassium-free".
3. How to use Infanrix-IPV vaccine
When Infanrix-IPV vaccine is administered
- The doctor or nurse will inform when the child should receive the vaccine. This depends on official recommendations.
How Infanrix-IPV vaccine is administered
- The child will receive one dose of the vaccine.
- Infanrix-IPV vaccine is always administered intramuscularly.
- The vaccine is usually administered in the arm muscle. However, in small children, it may be administered in the thigh.
- The vaccine should never be administered intravascularly.
In case of any further doubts about the use of this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them. The vaccine can cause the following side effects:
Allergic reactions
In case of an allergic reaction, the doctor should be contacted immediately. Symptoms may include:
- skin rash, which may be itchy or blistering
- swelling of the eyes and face
- difficulty breathing and swallowing
- a sudden drop in blood pressure
- loss of consciousness Such reactions usually occur soon after vaccination. The doctor should be contacted immediately if symptoms occur after leaving the doctor's office. Allergic reactions are very rare (less than 1 in 10,000 doses of the vaccine).
The doctor should be contacted immediately in case of the following symptoms:
- collapse
- loss of consciousness
- lack of awareness
- seizures
The doctor should be contacted immediately in case of the above symptoms. These side effects have occurred after administration of other pertussis vaccines. They usually occur within 2 to 3 days of vaccination.
Other side effects include:
Very common(more than 1 in 10 doses of the vaccine): sleepiness, headache, loss of appetite, high fever (38°C and higher), pain, redness, and swelling at the injection site, unusual crying, irritability, restlessness.
Common(less than 1 in 10 doses of the vaccine): diarrhea, nausea, vomiting, high fever (39.5°C and higher), malaise, hard lump at the injection site, weakness.
Uncommon(less than 1 in 100 doses of the vaccine): skin allergies or rash.
Rare(less than 1 in 1,000 doses of the vaccine): swelling of the lymph nodes in the neck, armpits, or groin (lymphadenopathy), cough or bronchitis, itching, lumpy rash (hives).
Very rare(less than 1 in 10,000 doses of the vaccine): bleeding or easy bruising (thrombocytopenia), longer than normal intervals between breaths (apnea), swelling of the face, lips, tongue, or throat that may cause difficulty swallowing or breathing (angioedema), blisters at the injection site. Booster doses of Infanrix-IPV vaccine may increase the risk of reactions at the injection site. Some of these reactions may affect the entire limb into which the vaccine was administered. These reactions usually start within 48 hours of vaccination and resolve within 4 days.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: 22 49 21 301, Fax: 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Infanrix-IPV vaccine
- The vaccine should be stored out of sight and reach of children.
- Store in a refrigerator (2°C to 8°C).
- Store in the original packaging to protect from light.
- Do not freeze. Freezing destroys the vaccine.
- Do not use this medicine after the expiry date stated on the carton after (EXP). The expiry date refers to the last day of the month stated.
- Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Infanrix-IPV vaccine contains
- The active substances are: diphtheria toxoid not less than 30 IU, tetanus toxoid not less than 40 IU, Bordetella pertussisantigens, pertussis toxoid 25 micrograms, filamentous hemagglutinin 25 micrograms, pertactin 8 micrograms, inactivated poliovirus type 1 (Mahoney strain) 40 antigen units, type 2 (MEF-1 strain) 8 antigen units, type 3 (Saukett strain) 32 antigen units, adsorbed on aluminum hydroxide, hydrated 0.5 milligrams, grown in VERO cell culture
Aluminum hydroxide acts as an adjuvant in this vaccine. Adjuvants are substances that are part of some vaccines and are intended to accelerate, enhance, and/or prolong the protective effect of the vaccine.
- Other ingredients are: sodium chloride, Medium 199 (containing amino acids (including phenylalanine), mineral salts (including sodium and potassium), vitamins (including para-aminobenzoic acid), and other substances), water for injections.
What Infanrix-IPV vaccine looks like and what the packaging contains
- Infanrix-IPV is a suspension for injection in a pre-filled syringe (0.5 ml).
- The suspension is a white, slightly milky liquid.
- Infanrix-IPV vaccine is available in a 1-dose pre-filled syringe, in packs of 1 or 10, with or without needles.
- Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals S.A., rue de l’Institut 89, 1330 Rixensart, Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Cyprus, Greece, Portugal: Infanrix Tetra
Estonia, Finland, Sweden: INFANRIX POLIO
France: INFANRIXTETRA
Hungary: INFANRIX IPV
Ireland: IPV Infanrix
Italy: POLIOINFANRIX
Latvia: Infanrix polio suspensija injekcijām pilnšļircē
Lithuania: Infanrix Polio injekcinė suspensija užpildytame švirkšte
Norway, Slovakia: Infanrix Polio
Poland: INFANRIX-IPV
Spain: INFANRIX-IPV SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Date of last revision of the leaflet: 11/2024
Other sources of information
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
-------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
During storage, a white sediment and a clear liquid may form above. This is a normal phenomenon and does not reduce the effectiveness of the vaccine.
Before administration, the pre-filled syringe should be shaken vigorously to obtain a homogeneous, cloudy, white suspension.
The suspension should be visually inspected for any foreign particles and/or changes in the physical appearance of the vaccine. If any are found, the vaccine should be discarded.
Instructions for the pre-filled syringe
Disposal
Any unused product or waste material should be disposed of in accordance with local regulations.
The pre-filled syringe should be held by the body, not by the plunger.
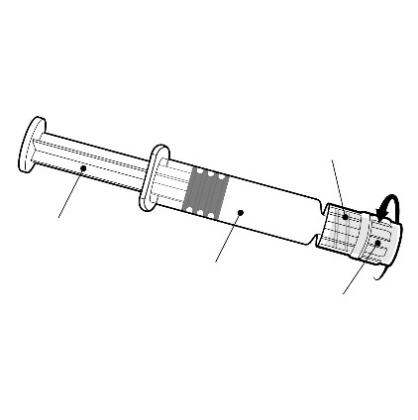
Needle hub
The needle hub should be unscrewed by rotating it counterclockwise.
Plunger
Body
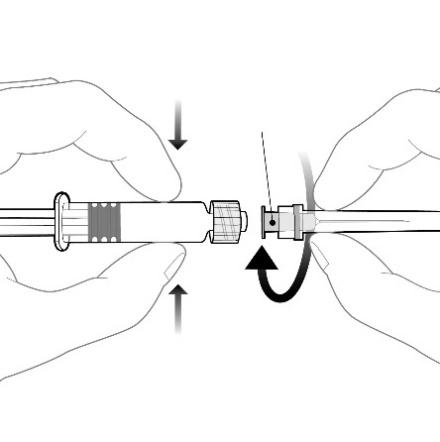
The needle should be attached to the pre-filled syringe by connecting the needle hub to the Luer Lock adapter and rotating it a quarter turn clockwise until the needle is locked.
The plunger should not be withdrawn from the body of the pre-filled syringe. If this happens, the vaccine should not be administered.
Needle hub
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGlaxoSmithKline Biologicals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Infanrix-ipvDosage form: Suspension, 0.5 mlActive substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: Sanofi-Aventis Zrt.Prescription requiredDosage form: Suspension, 0.5 ml (1 dose)Active substance: diphtheria-pertussis-poliomyelitis-tetanusManufacturer: GlaxoSmithKline Biologicals S.A.Prescription requiredDosage form: Powder, 32 antigen units D + 10 mcg + not less than 40 IU + 8 antigen units D + 40 antigen units D + not less than 30 IU + 25 mcg + 25 mcgActive substance: diphtheria-pertussis-poliomyelitis-tetanusPrescription required
Alternatives to Infanrix-ipv in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Infanrix-ipv in Испания
Alternative to Infanrix-ipv in Украина
Online doctors for Infanrix-ipv
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Infanrix-ipv – subject to medical assessment and local rules.














