
Iberogast

Ask a doctor about a prescription for Iberogast

How to use Iberogast
Package Leaflet: Information for the Patient
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Iberogast, Oral Liquid
Iberogast and Иберогаст are the same trade names for the same medicine, written in Polish and Bulgarian.
Read the Leaflet Carefully Before Using the Medicine, as it Contains Important Information for the Patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If after 7 days there is no improvement or the patient feels worse, they should contact their doctor.
Table of Contents of the Leaflet
- 1. What is Iberogast and what is it used for
- 2. Important information before using Iberogast
- 3. How to use Iberogast
- 4. Possible side effects
- 5. How to store Iberogast
- 6. Package contents and other information
1. What is Iberogast and what is it used for
Iberogast is a herbal medicine used to treat functional gastrointestinal disorders related to motility (passage) of the digestive tract, such as functional dyspepsia (indigestion) or irritable bowel syndrome. These disorders are mainly characterized by abdominal and intestinal cramps, abdominal pain, feeling of fullness, bloating, nausea, and heartburn. Iberogast alleviates the feeling of fullness, bloating, and reduces stomach and intestinal cramps. It has anti-inflammatory, carminative, antioxidant, and antibacterial properties.
2. Important information before using Iberogast
When not to use Iberogast
If the patient is allergic to any of the ingredients of this medicine. It is not recommended for children under 6 years of age. In children under 6 years of age, any abdominal pain should be consulted with a doctor. If the patient has or has had liver disease or is taking medicines that, according to the patient leaflet, may cause side effects in the form of liver damage. In case of any doubts, a doctor or pharmacist should be consulted.
Warnings and precautions
If the patient experiences yellowing of the skin or eyes, dark urine, pale stools, or abdominal pain, they should immediately stop using Iberogast and consult a doctor. These may be symptoms of liver damage. Patients with known liver disease should consult a doctor before starting to use Iberogast. If after 7 days there is no improvement or the patient feels worse, they should contact their doctor to rule out other serious diseases.
Iberogast and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. No interactions are known.
Iberogast and alcohol
Due to the alcohol content, Iberogast is not intended for and should not be used by people with alcoholism. Iberogast contains ethanol (alcohol) at a volume concentration of approximately 31%. A single dose for adults (20 drops) contains up to 240 mg of ethanol, which corresponds to approximately 6.2 ml of beer or 2.6 ml of wine per dose. Children and people at high risk, including patients with liver disease or epilepsy, should consult a doctor before using Iberogast.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine. As a precaution, it is recommended to avoid using Iberogast during pregnancy.
Driving and using machines
A single dose for adults contains up to 240 mg of alcohol. If Iberogast is used as recommended, it should not have a significant effect on the ability to drive vehicles and operate machines (see "Iberogast and alcohol").
3. How to use Iberogast
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. In case of doubts, a doctor or pharmacist should be consulted. Instructions for the first use of the dropper
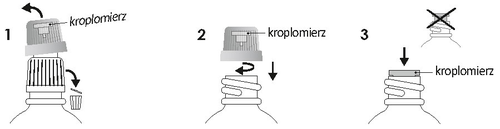
Unscrew the white and green cap. Screw the green cap tightly to place the dropper in the bottle. Make sure the dropper is well inserted. Throw away the white part of the cap. When dosing, hold the bottle with the dropper at a 45° angle. After use, close the green cap tightly. Oral administration. If the doctor does not recommend otherwise, Iberogast should be taken in a small amount of liquid before or during meals.
- Adults and adolescents over 12 years: 3 times a day, 20 drops each time,
- Children from 6 to 12 years: 3 times a day, 15 drops each time,
- Children under 6 years: not recommended.
Duration of use:
If after 7 days of using the medicine the symptoms do not improve, the patient should consult a doctor about their cause.
Shake before use.
The presence of sediment or cloudiness does not affect the efficacy of Iberogast liquid.
Using more than the recommended dose of Iberogast
There are no reports of acute overdose. However, the alcohol content should be considered. Accidental ingestion of one or two doses of Iberogast more than recommended, i.e., a total of 40 to 60 drops, usually does not lead to negative consequences. In case of ingestion of significantly larger doses, medical help should be sought.
Missing a dose of Iberogast
In case of missing a dose of Iberogast, the patient should wait until the next dose is due and then take the prescribed dose. A double dose should not be taken to make up for a missed dose.
4. Possible side effects
Like all medicines, Iberogast can cause side effects, although not everybody gets them. In very rare cases (less than 1 in 10,000), allergic reactions such as skin reactions, itching, or shortness of breath may occur. Unknown frequency: cases of liver damage (increased liver enzyme values, drug-induced jaundice, hepatitis, and liver failure) have been reported; if symptoms such as yellowing of the skin or eyes, dark urine, and pale stools occur, the patient should immediately stop taking Iberogast and consult a doctor.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Iberogast
Store in a temperature below 25°C. Store in a place out of sight and reach of children. Shelf life after first opening the bottle: 8 weeks. Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month. Medicines should not be disposed of via wastewater or household waste containers. A pharmacist should be asked how to dispose of medicines that are no longer used. This will help protect the environment.
6. Package contents and other information
What Iberogast contains
100 ml of liquid contains: Iberis amara herbae extractum(extract of bitter candytuft herb) (1:1.5-2.5) 15.0 ml, extraction solvent - ethanol 50% (v/v), Angelicae radicis extractum(extract of angelica root) (1:2.5-3.5) 10.0 ml, extraction solvent - ethanol 30% (v/v), Matricariae flos extractum(extract of chamomile flower) (1:2-4) 20.0 ml, extraction solvent - ethanol 30% (v/v), Carvi fructus extractum(extract of caraway fruit) (1:2.5-3.5) 10.0 ml, extraction solvent - ethanol 30% (v/v), Silybi mariani fructus extractum(extract of milk thistle fruit) (1:2.5-3.5) 10.0 ml, extraction solvent - ethanol 30% (v/v), Melissae folii extractum(extract of lemon balm leaves) (1:2.5-3.5) 10.0 ml, extraction solvent - ethanol 30% (v/v), Menthae piperitae folii extractum(extract of peppermint leaves) (1:2.5-3.5) 5.0 ml, extraction solvent - ethanol 30% (v/v), Chelidonii herbae extractum(extract of celandine herb) (1:2.5-3.5) 10.0 ml, extraction solvent - ethanol 30% (v/v), Liquiritiae radicis extractum(extract of licorice root) (1:2.5-3.5) 10.0 ml, extraction solvent - ethanol 30% (v/v). Iberogast contains 29.5-32.6% (v/v) ethanol.
What Iberogast looks like and what the package contains
Bottle made of dark glass with a dropper and screw cap, in a cardboard box. The package contains 20 ml of Iberogast liquid. For more detailed information, the marketing authorization holder or parallel importer should be contacted.
Marketing authorization holder in Bulgaria, the country of export:
Bayer Bulgaria EOOD, Rezbarska street No 5, 1510 Sofia, Bulgaria
Manufacturer:
STEIGERWALD Arzneimittelwerk GmbH, Havelstrasse 5, 64295 Darmstadt, Germany
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź, Authorization number in Bulgaria, the country of export: 20060714
Parallel import authorization number: 212/16
Date of leaflet approval: 15.01.2024
[Information about the trademark]
- Country of registration
- Prescription requiredNo
- Marketing authorisation holder (MAH)Bayer Bulgaria EOOD
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Iberogast
Online doctors for Iberogast
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Iberogast – subject to medical assessment and local rules.














