

Helipico

Ask a doctor about a prescription for Helipico

How to use Helipico
Package Leaflet: Information for the Patient
HeliPico, 27.78 mg/5 ml, Syrup
Hederae helicis folii extractum siccum
Read the Package Leaflet Carefully Before Using the Medication, as it Contains Important Information for the Patient.
This Medication Should Always be Used Exactly as Described in this Package Leaflet for the Patient or as Advised by a Doctor or Pharmacist.
- This Package Leaflet Should be Kept in Case it Needs to be Read Again.
- If Advice or Additional Information is Needed, a Pharmacist Should be Consulted.
- If the Patient Experiences any Undesirable Effects, Including any Possible Undesirable Effects not Listed in this Package Leaflet, the Doctor or Pharmacist Should be Informed. See Section 4.
- If There is no Improvement or the Patient Feels Worse After 7 Days, a Doctor Should be Consulted.
Package Leaflet Contents
- 1. What HeliPico is and What it is Used for
- 2. Important Information Before Taking HeliPico
- 3. How to Take HeliPico
- 4. Possible Undesirable Effects
- 5. How to Store HeliPico
- 6. Package Contents and Other Information
1. What HeliPico is and What it is Used for
HeliPico Syrup Contains Dry Extract of Ivy Leaves.
Indications for Use
HeliPico Syrup is a Herbal Medication Used as an Expectorant in the Case of Productive (Wet) Cough.
2. Important Information Before Taking HeliPico
When Not to Take HeliPico:
- If the Patient is Allergic to Ivy Leaves, to Other Plants of the Araliaceae Family or to Plants of the Apiaceae Family (formerly Umbelliferae), such as Anise, Caraway, Celery, Coriander, Dill, or to Anethole, or to any of the Other Ingredients of this Medication (listed in Section 6);
- In Children Under 2 Years of Age Due to the Risk of Worsening Respiratory Symptoms.
Warnings and Precautions
Before Starting to Take the Medication, the Doctor or Pharmacist Should be Consulted.
Persistent or Recurring Cough in Children Aged 2 to 4 Years Requires a Medical Diagnosis Before Starting Therapy.
In Case of Symptoms of Shortness of Breath, Fever, or Purulent Sputum, a Doctor or Pharmacist Should be Consulted.
Concomitant Use of Cough Medications, such as Codeine or Dextromethorphan, is not Recommended Without Consulting a Doctor.
Caution is Advised when Using in Patients with Gastric Mucosa Inflammation or Gastric Ulcer.
Children
The Medication is Contraindicated in Children Under 2 Years of Age.
HeliPico and Other Medications
The Doctor or Pharmacist Should be Informed About all Medications Currently or Recently Taken by the Patient, as well as any Medications Planned to be Taken.
No Interactions have been Reported so far.
Pregnancy, Breastfeeding, and Fertility
There are no Data on the Use of Ivy Leaf Preparations in Pregnant and Breastfeeding Women. HeliPico is not Recommended During Pregnancy and Breastfeeding.
There are no Data on the Effect of the Medication on Fertility.
Driving and Operating Machines
No Studies have been Conducted on the Effect of the Medication on Driving and Operating Machines.
HeliPico Contains Sorbitol (E420), Propylene Glycol (E1520), Macrogol Hydroxystearate
The Medication Contains 4200 mg of Non-Crystallizing Liquid Sorbitol, which Corresponds to 3024 mg of Sorbitol (E420) per 5 ml of Syrup, and is Equivalent to 0.252 Bread Units.
Sorbitol is a Source of Fructose. If the Patient (or their Child) has Previously been Diagnosed with Intolerance to some Sugars or has been Diagnosed with Hereditary Fructose Intolerance, a Rare Genetic Disorder in which the Patient's Body does not Break Down Fructose, the Patient Should Consult a Doctor Before Taking the Medication or Giving it to their Child.
Sorbitol may Cause Gastrointestinal Discomfort and may have a Mild Laxative Effect.
The Medication Contains 150.6 mg of Propylene Glycol (E1520) per 5 ml of Syrup.
The Medication Contains Macrogol Hydroxystearate. The Medication may Cause Nausea and Diarrhea.
This Medication Contains 0.7 mg of Alcohol per 5 ml of Syrup. The Amount of Alcohol in each 5 ml of this Medication is Equivalent to Less than 1 ml of Beer or Less than 1 ml of Wine. The Small Amount of Alcohol in this Medication will not have Noticeable Effects.
Instructions for Diabetics
HeliPico Syrup does not Contain Sugar and can be Used by Diabetics.
3. How to Take HeliPico
This Medication Should Always be Taken Exactly as Described in this Package Leaflet for the Patient or as Advised by a Doctor or Pharmacist. In Case of Doubt, a Doctor or Pharmacist Should be Consulted.
The Medication is Administered Orally. A Dosage Device in the Form of an Oral Syringe with a Gradation Facilitating Dosing is Attached to the Packaging.
Unless Otherwise Advised by a Doctor, the Following Dosage Regimen Should be Used:
Adolescents from 12 Years of Age, Adults, and Elderly Patients: 2 to 3 Times a Day, 5 ml (full Oral Syringe) Each Time, which Corresponds to a Daily Dose of 55.56 mg to 83.34 mg of Dry Extract.
Children from 6 to 11 Years of Age: 3 Times a Day, 2.5 ml of Medication (½ Oral Syringe), which Corresponds to a Daily Dose of 41.67 mg of Dry Extract.
Children from 2 to 5 Years of Age: 3 Times a Day, 1.6 ml of Medication (⅓ Oral Syringe), which Corresponds to a Daily Dose of 26.67 mg of Dry Extract.
Use in Children Under 2 Years of Age is Contraindicated.
If There is no Improvement or the Patient Feels Worse After 7 Days, a Doctor Should be Consulted.
Instructions for Using the Dosage Device in the Form of an Oral Syringe
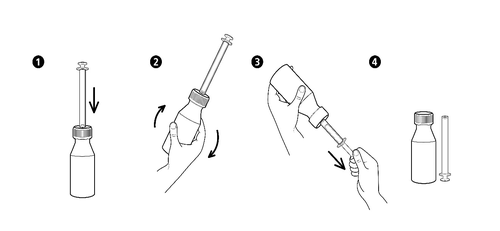
- 1. Unscrew the Bottle Cap (press Down and Turn in the Opposite Direction to the Clockwise Direction).
- 2. Insert the Oral Syringe into the Adapter Located in the Bottle Neck.
- 3. To Fill the Oral Syringe, the Bottle Should be Turned Upside Down and then the Plunger of the Oral Syringe Should be Carefully Moved Down, Drawing the Syrup to the Desired Mark on the Gradation.
- 4. The Bottle Should be Turned Back to its Original Position and the Oral Syringe Should be Carefully Removed from the Bottle.
- 5. The Tip of the Oral Syringe Should be Placed in the Child's Mouth and then, by Slowly Pressing the Plunger, the Contents of the Oral Syringe Should be Carefully Emptied.
- 6. After Use, the Bottle Should be Closed by Screwing the Cap and the Oral Syringe Should be Washed and Dried.
Overdose of HeliPico
Overdose may Cause Nausea, Vomiting, Diarrhea, and Increased Motor Activity.
In Case of Overdose, a Doctor Should be Consulted Immediately, who will Prescribe the Appropriate Symptomatic Treatment.
Missed Dose of HeliPico
A Double Dose Should not be Taken to Make up for a Missed Dose. If a Dose is Missed and it is Almost Time for the Next Dose, the Missed Dose Should not be Taken.
Stopping HeliPico Treatment
In Case of any Further Doubts Regarding the Use of this Medication, a Doctor or Pharmacist Should be Consulted.
4. Possible Undesirable Effects
Like all Medications, this Medication can Cause Undesirable Effects, although not Everybody will Experience them.
Allergic Reactions (hives, Rash, Shortness of Breath), Nausea, Vomiting, Diarrhea may Occur.
The Frequency of Undesirable Effects is Unknown.
Reporting Undesirable Effects
If any Undesirable Effects Occur, including any Undesirable Effects not Listed in this Package Leaflet, a Doctor or Pharmacist Should be Consulted. Undesirable Effects can be Reported Directly to the Department of Monitoring of Undesirable Effects of Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181C 02-222 Warsaw Tel.: +48 22 49 21 301 Fax: +48 22 49 21 309 Website: https://smz.ezdrowie.gov.pl Undesirable Effects can also be Reported to the Marketing Authorization Holder. By Reporting Undesirable Effects, more Information can be Collected on the Safety of the Medication.
5. How to Store HeliPico
The Medication Should be Stored in a Place Invisible and Inaccessible to Children.
Do not Store at a Temperature Above 25°C.
Store in the Original Packaging. Store the Bottle Tightly Closed.
Do not Use this Medication After the Expiration Date (EXP) Stated on the Packaging.
The Shelf Life of the Medication After Opening the Bottle is 6 Months.
Medications Should not be Disposed of in the Drain or Household Waste Containers. A Pharmacist Should be Asked how to Dispose of Unused Medications. This will Help Protect the Environment.
6. Package Contents and Other Information
What HeliPico Contains
- The Active Substance of the Medication is Dry Extract of Ivy Leaves (Hederae helicis folii extractum siccum). 100 ml of Syrup Contains 555.6 mg of Dry Extract of Hedera helix L., folium (4-8:1). Extraction Solvent: Ethanol 30% (m/m).
- The Other Ingredients are: Propylene Glycol, Non-Crystallizing Liquid Sorbitol (E420), Macrogol Hydroxystearate, Potassium Sorbate (E202), Citric Acid Monohydrate, Xanthan Gum, Anise Flavor (contains, among others, Ethanol, Propylene Glycol, Propanol, Natural Anise Oil), Purified Water.
What HeliPico Looks Like and What the Packaging Contains
Brown PET Bottle with Adapter made of Polyethylene with Polyethylene Cap with HDPE, with a Security Ring, with a Child-Resistant Closure System, with a Dosage Device in the Form of an Oral Syringe, in a Cardboard Box.
Slight Opalescence of the Syrup and Sediment Formed are Due to the Presence of Plant Ingredients. This does not Affect the Medication's Action.
1 Bottle of 100 ml
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Polpharma S.A. Pharmaceutical Works ul. Pelplińska 19, 83-200 Starogard Gdański tel. +48 22 364 61 01
Manufacturer
Polpharma S.A. Pharmaceutical Works Medana Branch in Sieradz ul. Władysława Łokietka 10, 98-200 Sieradz tel. 43 829 92 00
Date of the Last Update of the Package Leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterZakłady Farmaceutyczne POLPHARMA S.A. Oddział Medana w Sieradzu
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HelipicoDosage form: Syrup, 700 mg/100 mlActive substance: Hederae helicis foliumManufacturer: Krewel Meuselbach GmbHPrescription not requiredDosage form: Syrup, 40 mg/5 mlActive substance: Hederae helicis foliumManufacturer: Krewel Meuselbach GmbHPrescription not requiredDosage form: Tablets, 52.5 mgActive substance: Hederae helicis foliumManufacturer: Wrocławskie Zakłady Zielarskie "Herbapol" S.A.Prescription not required
Alternatives to Helipico in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Helipico in Spain
Alternative to Helipico in Ukraine
Online doctors for Helipico
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Helipico – subject to medical assessment and local rules.














