

Hedelix

Ask a doctor about a prescription for Hedelix

How to use Hedelix
PATIENT INFORMATION LEAFLET
(measuring spoon )
Leaflet enclosed with the packaging: information for the patient
Hedelix, 40 mg/5 ml, syrup
(Hederae helicis folii extractum spissum)
Thick extract of ivy leaves
Before taking the medicine, carefully read the contents of the leaflet, as it contains
important information for the patient.
This medicine should always be used exactly as described in the patient information leaflet or according to the doctor's or pharmacist's instructions.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, the pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. See section 4.
- If after 7 days there is no improvement or the patient feels worse, the doctor should be consulted.
Table of contents of the leaflet
- 1. What is Hedelix and what is it used for
- 2. Important information before taking Hedelix
- 3. How to take Hedelix
- 4. Possible side effects
- 5. How to store Hedelix
- 6. Contents of the packaging and other information
1. What is Hedelix and what is it used for
Hedelix is a syrup containing a thick extract of common ivy leaves (Hederae helicis folii extractum spissum) as the active substance.
A herbal medicinal product used as an expectorant in productive (so-called wet) cough.
If after 7 days there is no improvement or the patient feels worse, the doctor should be consulted.
2. Important information before taking Hedelix
When not to take Hedelix:
- if the patient is allergic to ivy leaf extract or plants of the Araliaceae family or any of the other ingredients of this medicine (listed in section 6).
- Do not use in children under 2 years of age because in this age group, respiratory symptoms may worsen (exacerbation of disease symptoms).
Warnings and precautions
Consult a doctor or pharmacist before taking the medicine.
Hedelix should not be taken in disorders of arginine biosynthesis (disorder of nitrogen metabolism in the urea cycle).
Persistent or recurrent cough in children aged 2 to 4 years requires diagnosis before taking the medicine.
If shortness of breath, fever, or purulent sputum occurs, consult a doctor or pharmacist.
Concomitant use with opioid cough suppressants, such as codeine or dextromethorphan, is not recommended without medical supervision.
Caution is recommended in patients with gastritis or gastric ulcers.
Due to the content of plant extract, Hedelix syrup may form a sediment in the bottle. The sediment may cause clouding of the solution, and the taste may undergo slight changes.
Other medicines and Hedelix
Tell the doctor or pharmacist about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
No interactions with other medicines have been reported.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before taking this medicine.
The safety of use during pregnancy and lactation has not been established. Due to the lack of sufficient data, use during pregnancy and lactation is not recommended.
Driving and using machines
It has no influence.
Hedelix contains sorbitol
The medicine contains 1.75 g of sorbitol per 5 ml (which corresponds to 0.44 g of fructose) and is equivalent to approximately 0.15 carbohydrate units.
Sorbitol is a source of fructose. If the patient (or their child) has previously been diagnosed with intolerance to some sugars or has been diagnosed with hereditary fructose intolerance, a rare genetic disease in which the patient's body does not break down fructose, the patient should consult a doctor before taking the medicine or giving it to their child.
3. How to take Hedelix
This medicine should always be used exactly as described in the patient information leaflet or according to the doctor's or pharmacist's instructions.
To ensure accurate dosing, use the measuring spoon provided with the packaging.
Recommended dose:
Adolescents, adults, and the elderly:
5 ml 3 times a day.
Children aged 6-11 years:
2.5 ml 4 times a day.
Children aged 2-5 years:
2.5 ml 3 times a day.
Children under 2 years of age:
Do not use in children under 2 years of age (see section 2. Important information before taking Hedelix).
Duration of treatment
Hedelix can be used without medical supervision for 7 days.
If symptoms persist for more than a week, consult a doctor or pharmacist.
If the effect of Hedelix is too strong or too weak, consult a doctor or pharmacist.
Taking a higher dose of Hedelix than recommended
If one or two single doses are taken in excess of the recommended dose, it is unlikely to cause any symptoms.
Taking a significantly larger amount of the medicine may cause nausea, vomiting, diarrhea, and excitement.
In such cases, consult a doctor.
Missing a dose of Hedelix
Do not take a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following frequency classification has been used to determine the frequency of side effects:
very common (≥ 1/10);
common (≥ 1/100 to <1>uncommon (≥ 1/1,000 to <1>rare (≥ 1/10,000 to <1>very rare (<1>frequency not known (cannot be estimated from the available data).
Immune system disorders:
Frequency not known: allergic reactions (hives, skin rash, shortness of breath).
Gastrointestinal disorders:
Frequency not known: gastrointestinal disorders (nausea, vomiting, diarrhea).
If side effects occur that are not listed above, consult a doctor or pharmacist.
Reporting side effects
If any side effects occur, including any possible side effects not listed in the leaflet, inform the doctor or pharmacist.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warszawa, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Hedelix
Store the medicine out of sight and reach of children.
Store at a temperature below 25°C.
Do not use the medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the specified month.
Shelf life after first opening: 6 months
6. Contents of the packaging and other information
What Hedelix contains
- The active substance of the medicine is a thick extract of common ivy leaves (Hederae helicis folii extractum spissum). 100 ml of syrup contains 0.8 g of thick extract of Hedera helixL., folium(common ivy leaf) (2.2-2.9:1); extraction solvent: ethanol 96% (V/V); propylene glycol; water (45:2:53) m/m/m. The other ingredients are: macrogol glycerol hydroxystearate, anise oil, hydroxyethyl cellulose, sorbitol (70% solution) (E 420), propylene glycol, glycerol, purified water.
The medicine does not contain ethanol (alcohol).
What Hedelix looks like and what the packaging contains
Hedelix is a clear, yellowish-brown solution.
Hedelix is available in 100 ml and 200 ml packaging.
A measuring spoon is included with the packaging.
Marketing authorization holder:
Fortis Pharmaceuticals Sp. z o. o.
ul. Mickiewicza 29,
40-085 Katowice
Poland
Manufacturer:
Krewel Meuselbach GmbH
Krewelstrasse 2
- D- 53783 Eitorf
Germany
Date of last revision of the leaflet:
PATIENT INFORMATION LEAFLET
(Dosing syringe)
Leaflet enclosed with the packaging: information for the patient
Hedelix, 40 mg/5 ml, syrup
(Hederae helicis folii extractum spissum)
Thick extract of ivy leaves
Before taking the medicine, carefully read the contents of the leaflet, as it contains
important information for the patient.
This medicine should always be used exactly as described in the patient information leaflet or according to the doctor's or pharmacist's instructions.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, the pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, the doctor or pharmacist should be informed. See section 4.
- If after 7 days there is no improvement or the patient feels worse, the doctor should be consulted.
Table of contents of the leaflet
- 1. What is Hedelix and what is it used for
- 2. Important information before taking Hedelix
- 3. How to take Hedelix
- 4. Possible side effects
- 5. How to store Hedelix
- 6. Contents of the packaging and other information
1. What is Hedelix and what is it used for
Hedelix is a syrup containing a thick extract of common ivy leaves (Hederae helicis folii extractum spissum) as the active substance.
A herbal medicinal product used as an expectorant in productive (so-called wet) cough.
If after 7 days there is no improvement or the patient feels worse, the doctor should be consulted.
2. Important information before taking Hedelix
When not to take Hedelix:
- if the patient is allergic to ivy leaf extract or plants of the Araliaceae family or any of the other ingredients of this medicine (listed in section 6). Do not use in children under 2 years of age because in this age group, respiratory symptoms may worsen (exacerbation of disease symptoms).
Warnings and precautions
Consult a doctor or pharmacist before taking the medicine.
Hedelix should not be taken in disorders of arginine biosynthesis (disorder of nitrogen metabolism in the urea cycle).
Persistent or recurrent cough in children aged 2 to 4 years requires diagnosis before taking the medicine.
If shortness of breath, fever, or purulent sputum occurs, consult a doctor or pharmacist.
Concomitant use with opioid cough suppressants, such as codeine or dextromethorphan, is not recommended without medical supervision.
Caution is recommended in patients with gastritis or gastric ulcers.
Due to the content of plant extract, Hedelix syrup may form a sediment in the bottle. The sediment may cause clouding of the solution, and the taste may undergo slight changes.
Other medicines and Hedelix
Tell the doctor or pharmacist about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
No interactions with other medicines have been reported.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant, or plans to have a child, she should consult a doctor or pharmacist before taking this medicine.
The safety of use during pregnancy and lactation has not been established. Due to the lack of sufficient data, use during pregnancy and lactation is not recommended.
Driving and using machines
It has no influence.
Hedelix contains sorbitol
The medicine contains 1.75 g of sorbitol per 5 ml (which corresponds to 0.44 g of fructose) and is equivalent to approximately 0.15 carbohydrate units.
Sorbitol is a source of fructose. If the patient (or their child) has previously been diagnosed with intolerance to some sugars or has been diagnosed with hereditary fructose intolerance, a rare genetic disease in which the patient's body does not break down fructose, the patient should consult a doctor before taking the medicine or giving it to their child.
3. How to take Hedelix
This medicine should always be used exactly as described in the patient information leaflet or according to the doctor's or pharmacist's instructions.
To ensure accurate dosing, use the dosing syringe provided with the packaging.
Recommended dose:
Adolescents, adults, and the elderly:
5 ml 3 times a day.
Children aged 6-11 years:
2.5 ml 4 times a day.
Children aged 2-5 years:
2.5 ml 3 times a day.
Children under 2 years of age:
Do not use in children under 2 years of age (see section 2. Important information before taking Hedelix).
How to use the dosing syringe:
The accurate graduation necessary for measuring the correct dose is printed on the dosing syringe
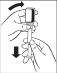

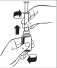
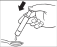

- 1.
- 1. Unscrew the bottle cap. Connect the dosing syringe to the open bottle. Gently press
- 2.
- 3.
- 4.
the syringe into the bottle until the syringe is locked in place. At this time, the plunger should be inside the syringe.
- 2. Carefully turn the bottle upside down and draw the correct amount of syrup by gently moving the syringe plunger to the correct position on the graduation.
- 3. Turn the bottle back to its original position and remove the syringe by gently twisting it. Close the bottle after taking the dose.
- 4. The syrup can be administered directly by emptying the dosing syringe into the mouth onto the cheek or using a spoon.
- 5. After taking the dose, wash and dry each part of the syringe.
Hedelix should be taken undiluted. It is recommended to drink the syrup with a significant amount of liquid, preferably a glass of water.
Duration of treatment
Hedelix can be used without medical supervision for 7 days.
If symptoms persist for more than a week, consult a doctor or pharmacist.
If the effect of Hedelix is too strong or too weak, consult a doctor or pharmacist.
Taking a higher dose of Hedelix than recommended
If one or two single doses are taken in excess of the recommended dose, it is unlikely to cause any symptoms.
Taking a significantly larger amount of the medicine may cause nausea, vomiting, diarrhea, and excitement.
In such cases, consult a doctor.
Missing a dose of Hedelix
Do not take a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following frequency classification has been used to determine the frequency of side effects:
very common (≥ 1/10);
common (≥ 1/100 to <1>uncommon (≥ 1/1,000 to <1>rare (≥ 1/10,000 to <1>very rare (<1>frequency not known (cannot be estimated from the available data).
Immune system disorders:
Frequency not known: allergic reactions (hives, skin rash, shortness of breath).
Gastrointestinal disorders:
Frequency not known: gastrointestinal disorders (nausea, vomiting, diarrhea).
If side effects occur that are not listed above, consult a doctor or pharmacist.
Reporting side effects
If any side effects occur, including any possible side effects not listed in the leaflet, inform the doctor or pharmacist.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warszawa, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Hedelix
Store the medicine out of sight and reach of children.
Store at a temperature below 25°C.
Do not use the medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the specified month.
Shelf life after first opening: 6 months
6. Contents of the packaging and other information
What Hedelix contains
- The active substance of the medicine is a thick extract of common ivy leaves (Hederae helicis folii extractum spissum). 100 ml of syrup contains 0.8 g of thick extract of Hedera helixL. folium(common ivy leaf) (2.2-2.9:1); extraction solvent: ethanol 96% (V/V); propylene glycol; water (45:2:53) m/m/m.
The other ingredients are: macrogol glycerol hydroxystearate, anise oil, hydroxyethyl cellulose, sorbitol (70% solution) (E 420), propylene glycol, glycerol, purified water.
The medicine does not contain ethanol (alcohol).
What Hedelix looks like and what the packaging contains
Hedelix is a clear, yellowish-brown solution.
Hedelix is available in 100 ml and 200 ml packaging.
A dosing syringe is included with the packaging.
Marketing authorization holder:
Fortis Pharmaceuticals Sp. z o. o.
ul. Mickiewicza 29,
40-085 Katowice
Poland
Manufacturer:
Krewel Meuselbach GmbH
Krewelstrasse 2
- D- 53783 Eitorf Germany
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterKrewel Meuselbach GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HedelixDosage form: Syrup, 700 mg/100 mlActive substance: Hederae helicis foliumManufacturer: Krewel Meuselbach GmbHPrescription not requiredDosage form: Tablets, 52.5 mgActive substance: Hederae helicis foliumManufacturer: Wrocławskie Zakłady Zielarskie "Herbapol" S.A.Prescription not requiredDosage form: Syrup, 26.6 mg/5 mlActive substance: Hederae helicis foliumManufacturer: Wrocławskie Zakłady Zielarskie "Herbapol" S.A.Prescription not required
Alternatives to Hedelix in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Hedelix in Испания
Alternative to Hedelix in Украина
Online doctors for Hedelix
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Hedelix – subject to medical assessment and local rules.














