
Havrix Adult

Ask a doctor about a prescription for Havrix Adult

How to use Havrix Adult
Patient Information Leaflet: User Manual
Havrix Adult, Suspension for Injection
Hepatitis A vaccine, inactivated, adsorbed
Read the leaflet carefully before using the vaccine, as it contains
important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- This vaccine has been prescribed specifically for this person. Do not pass it on to others.
- If the patient experiences any side effects, including any not listed in this leaflet, tell the doctor, pharmacist, or nurse. See section 4.
The content of this leaflet has been written with the assumption that it will be read by the person receiving the vaccine. However, since this vaccine may be administered to adolescents from 16 years of age, it is possible that the content of the leaflet will be read by the child's parent or guardian.
Table of Contents of the Leaflet:
- 1. What is Havrix Adult vaccine and what is it used for
- 2. Important information before using Havrix Adult vaccine
- 3. How to use Havrix Adult vaccine
- 4. Possible side effects
- 5. How to store Havrix Adult vaccine
- 6. Contents of the pack and other information
1. What is Havrix Adult vaccine and what is it used for
What is Havrix Adult vaccine used for
Havrix Adult vaccine is used to prevent hepatitis A virus infection (HAV) in people aged 16 and above who are at risk of infection with the hepatitis A virus (HAV).
What is hepatitis A:
‐
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV).
‐
The HAV virus is spread from person to person or through contact with contaminated water, food, and drinks.
‐
The symptoms of hepatitis A range from mild to severe and may include fever, feeling unwell, loss of appetite, diarrhea, nausea, discomfort in the abdominal cavity, dark urine, and jaundice (yellowing of the eyes and skin). Most patients recover completely, but sometimes the disease can be severe and require hospitalization, and in rare cases, it can lead to acute liver failure.
How Havrix Adult works:
‐
Havrix Adult helps the body produce its own protection (antibodies) against the virus.
‐
Like all vaccines, Havrix Adult may not fully protect all vaccinated people.
2. Important information before using Havrix Adult vaccine
When not to use Havrix Adult vaccine
‐
If the patient is allergic to the active substance or any of the other ingredients of this vaccine (listed in section 6) or to neomycin or formaldehyde,
‐
If the patient has ever had an allergic reaction to any hepatitis A vaccine.
Symptoms of an allergic reaction may include an itchy skin rash, difficulty breathing, swelling of the face or tongue.
Do not give Havrix Adult vaccine if any of the above situations apply to the patient. In case of doubt before administering Havrix Adult vaccine, consult a doctor, pharmacist, or nurse.
Warnings and precautions
Before administering Havrix Adult vaccine, discuss with the doctor if:
‐
The patient has a severe infection with a high temperature (fever). In this case, vaccination will be postponed. Mild infections, such as a cold, should not be a contraindication to vaccination, but first, tell the doctor,
‐
The patient has a weakened immune system due to disease and/or medication. The doctor will decide if additional doses are needed,
‐
The patient experiences bleeding problems or easy bruising.
After or even before administering each injection vaccine, fainting may occur. Inform the doctor, pharmacist, or nurse if the patient has ever fainted when receiving an injection.
Havrix Adult vaccine and other medicines
Tell the doctor, pharmacist, or nurse about all medicines the patient is taking or has recently taken, as well as medicines the patient plans to take and recently received vaccines.
Havrix Adult vaccine can be administered at the same time as some other vaccines and immunoglobulins, but the injection sites must be different.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant, or plans to have a child, consult a doctor, pharmacist, or nurse before using this vaccine.
Driving and using machines
Havrix Adult vaccine has no or negligible influence on the ability to drive and use machines.
Havrix Adult contains phenylalanine, sodium, and potassium
This vaccine contains 0.166 mg of phenylalanine per dose.
Phenylalanine may be harmful to patients with phenylketonuria, a rare genetic disorder in which phenylalanine accumulates in the body because the body does not eliminate it properly.
This vaccine contains less than 1 mmol (23 mg) of sodium per dose and less than 1 mmol (39 mg) of potassium per dose, i.e., the vaccine is considered "sodium and potassium-free".
3. How to use Havrix Adult vaccine
How is Havrix Adult vaccine administered
‐
Havrix Adult vaccine is administered by intramuscular injection, usually in the upper arm.
‐
In patients with low platelet count or severe bleeding disorders, Havrix Adult vaccine may be administered subcutaneously as an exception.
Dosage
‐
The patient will receive 1 dose of Havrix Adult vaccine (1 ml suspension) on the day agreed with the doctor or nurse.
‐
A second dose (booster dose) is recommended to be administered between 6 and 12 months after the first dose, but it can be administered up to 5 years after the first dose to ensure long-term protection.
Overdose of Havrix Adult vaccine
Overdose is very unlikely, as the vaccine is supplied in a vial or syringe with a single dose and is administered by a doctor or nurse. A few cases of accidental overdose have been reported, and the adverse reactions reported were similar to those reported with normal vaccination (listed in section 4).
If the next dose of Havrix Adult vaccine is missed
Contact the doctor, who will decide if another dose is needed.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Severe side effects
Tell the doctor immediately if any of the following severe side effects occur - urgent medical attention may be needed:
‐
Allergic reactions - symptoms may include local or widespread rash, which may itch or blister, swelling of the eyes and face, difficulty breathing or swallowing, sudden drop in blood pressure, and loss of consciousness.
These reactions may occur before leaving the doctor's office.
If any of the above severe side effects occur, tell the doctor immediately.
The following side effects have been reported during clinical trials with Havrix Adult:
Very common(may occur more often than 1 in 10 doses of the vaccine):
- headache
- pain and redness at the injection site
- fatigue
Common(may occur no more often than 1 in 10 doses of the vaccine):
- decreased appetite
- nausea (vomiting)
- vomiting
- diarrhea
- general feeling of being unwell
- fever ≥ 37.5°C
- swelling or hardening at the injection site
Uncommon(may occur no more often than 1 in 100 doses of the vaccine):
- upper respiratory tract infection
- stuffy nose or runny nose
- dizziness
- muscle pain, stiffness, unexplained by exercise
- flu-like symptoms, such as high temperature, sore throat, runny nose, cough, and chills
Rare(may occur no more often than 1 in 1000 doses of the vaccine):
- decreased or lost skin sensitivity to pain or touch
- tingling
- itching
- chills
The following side effects have been reported after Havrix Adult vaccine was marketed:
‐
seizures
‐
narrowing or blockage of blood vessels
‐
severe allergic reaction causing swelling of the face, tongue, or throat, which may cause difficulty swallowing or breathing
‐
hives, red, often itchy patches, which start on the limbs and sometimes on the face and rest of the body
‐
joint pain
Reporting side effects
If any side effects occur, including any not listed in this leaflet, tell the doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Havrix Adult vaccine
Keep the vaccine out of sight and reach of children.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original packaging to protect from light.
Do not use this vaccine after the expiry date stated on the packaging after "EXP". The expiry date refers to the last day of the month.
The "Lot" abbreviation means the batch number of the product.
6. Contents of the pack and other information
What Havrix Adult vaccine contains
- The active substance of Havrix Adult vaccine is:
Hepatitis A virus, strain HM175 (inactivated)
not less than 1440 ELISA units
propagated in human diploid cell culture MRC-5
adsorbed on hydrated aluminum hydroxide
0.5 mg Al
- The other ingredients are: polysorbate 20, amino acids for injection (including phenylalanine), sodium phosphate, potassium dihydrogen phosphate, sodium chloride, potassium chloride, water for injection.
What Havrix Adult vaccine looks like and contents of the pack
Havrix Adult is a slightly opaque, white suspension in a vial or pre-filled syringe.
Pack sizes:
1 vial of 1 ml in a cardboard box
1 pre-filled syringe with needle of 1 ml in a cardboard box
1 pre-filled syringe of 1 ml with attached needle in a cardboard box
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals S.A.
rue de l’Institut 89
1330 Rixensart, Belgium
For more detailed information, please contact the local representative of the marketing authorization holder:
GSK Services Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warsaw
tel. +48 (22) 576-90-00
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Czech Republic, Denmark, Estonia, Finland, Greece, Iceland, Italy | HAVRIX |
| Norway, Sweden | Havrix |
| Austria, Belgium, Hungary, Luxembourg, Netherlands | HAVRIX 1440 |
| Germany | Havrix 1440 |
| Bulgaria | ХАВРИКС 1440 инжекционна суспензия (доза за възрастни) |
| Cyprus | HAVRIX 1440 IU |
| France | HAVRIX 1440 U/1ml ADULTES |
| Ireland, Malta | HAVRIX MONODOSE |
| Latvia | HAVRIX 1440 ELISA units/ml vienības/ml suspensija injekcijām |
| Lithuania | Havrix 1440 ELISA vienetų/ml injekcinė suspensija |
| Poland | HAVRIX ADULT |
| Portugal | HAVRIX 1440 ADULTO |
| Romania | HAVRIX ADULT 1440 suspensie injectabilă |
| Slovak Republic | HAVRIX 1440 Dosis adulta |
| Slovenia | HAVRIX 1440 ELISA enot suspenzija za injiciranje |
Date of last revision of the leaflet:
08/2024
Information intended for healthcare professionals or healthcare workers only:
During storage, a white sediment and a clear, colorless liquid may appear at the bottom of the vial or pre-filled syringe. Before administration, the vaccine should be inspected for any foreign particles and/or physical changes.
Before use, shake the vial or pre-filled syringe to obtain a slightly opaque, white suspension.
Do not administer the vaccine if any changes in its appearance are observed.
| for adults | |
| Spain | HAVRIX 1440 suspensión inyectable en jeringa precargada |
Instructions for the pre-filled syringe
Hold the pre-filled syringe by the body, not by the plunger.
Remove the cap from the pre-filled syringe by twisting it in a counter-clockwise direction.
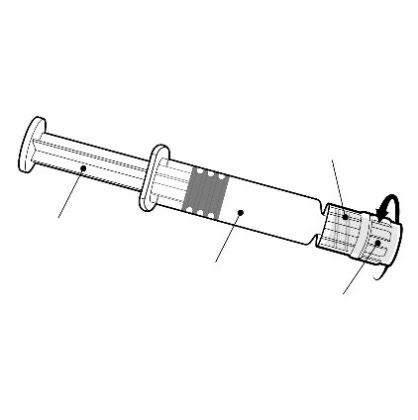
Luer Lock adapter
Plunger
Body
Cap
Attach the needle to the pre-filled syringe by connecting the needle cap to the Luer Lock adapter (Luer Lock Adaptor, LLA) and turning it a quarter turn in a clockwise direction until the needle is locked.
Do not pull the plunger out of the body of the pre-filled syringe. If this happens, do not administer the vaccine.
Disposal
Dispose of any unused product or waste in accordance with local regulations.
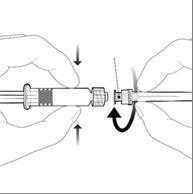
Needle cap
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGlaxoSmithKline Biologicals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Havrix AdultDosage form: Suspension, 160 ELISA antigen units of hepatitis A virus, strain GBM/0.5 ml; 1 dose (0.5 ml)Active substance: hepatitis A, inactivated, whole virusPrescription requiredDosage form: Suspension, at least 720 ELISA units of hepatitis A virus, strain HM175/0.5 ml; 1 dose (0.5 ml)Active substance: hepatitis A, inactivated, whole virusManufacturer: GlaxoSmithKline Biologicals S.A.Prescription requiredDosage form: Suspension, 25 U of hepatitis A virus, strain CR326F/0.5 ml; 1 dose (0.5 ml), for children and adolescentsActive substance: hepatitis A, inactivated, whole virusManufacturer: Merck Sharp & Dohme B.V.Prescription required
Alternatives to Havrix Adult in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Havrix Adult in Spain
Alternative to Havrix Adult in Ukraine
Online doctors for Havrix Adult
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Havrix Adult – subject to medical assessment and local rules.














