
Glucagen 1 mg Hipokit

Ask a doctor about a prescription for Glucagen 1 mg Hipokit

How to use Glucagen 1 mg Hipokit
Leaflet accompanying the packaging: information for the user
GlucaGen 1 mg HypoKit, 1 mg,
Powder and solvent for solution for injection
glucagon
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor. See section 4.
Table of contents of the leaflet
- 1. What is GlucaGen 1 mg HypoKit and what is it used for
- 2. Important information before using GlucaGen 1 mg HypoKit
- 3. How to use GlucaGen 1 mg HypoKit
- 4. Possible side effects
- 5. How to store GlucaGen 1 mg HypoKit
- 6. Contents of the pack and other information
1. What is GlucaGen 1 mg HypoKit and what is it used for
GlucaGen 1 mg HypoKit contains the active substance glucagon.
Glucagon is a natural hormone that acts in the human body in opposition to insulin. It facilitates the conversion of glycogen into glucose (sugar) in the liver. Then glucose is released into the bloodstream, which increases blood sugar levels.
Therapeutic indications (severe hypoglycaemia)
GlucaGen 1 mg HypoKit is intended for immediate use in emergency situations in children and adults with diabetes using insulin. It is used in cases of loss of consciousness (loss of consciousness) caused by very low blood sugar levels, known as severe hypoglycaemia. GlucaGen 1 mg HypoKit is used when the patient cannot take glucose orally.
Diagnostic indications
GlucaGen 1 mg HypoKit is used in adults to inhibit the motor activity of the gastrointestinal tract during diagnostic tests.
Information intended only for healthcare professionals, see the end of this leaflet.
2. Important information before using GlucaGen 1 mg HypoKit
When not to use GlucaGen 1 mg HypoKit
Warnings and precautions
Before starting treatment with GlucaGen 1 mg HypoKit, discuss it with your doctor, pharmacist or nurse.
Due to the instability of GlucaGen in solution, the medicine should be administered immediately after preparation and should not be administered as an intravenous infusion.
Important information:
- make sure that family members and people in the immediate vicinity are informed about the possibility of using GlucaGen 1 mg HypoKit. They should be informed about the need for immediate administration of GlucaGen 1 mg HypoKit in case of loss of consciousness (loss of consciousness) of the patient, when oral intake of carbohydrates is not possible;
- instruct family members and people in the immediate vicinity where the medicine is stored and how to use it. People providing assistance must act quickly - prolonged loss of consciousness can be harmful. These people should be trained in the use of GlucaGen 1 mg HypoKit before the need to use the medicine arises;
- the pre-filled syringe does not contain GlucaGen. Before administration, a solution must be prepared by mixing the water in the pre-filled syringe with the glucagon powder in the vial. Instruct family members and people in the immediate vicinity to follow the instructions that can be found in section 3: How to use GlucaGen 1 mg HypoKit;
- the prepared solution containing glucagon that has not been used should be discarded;
- after using GlucaGen 1 mg HypoKit, contact your doctor. Determine what caused the very low blood sugar level and how to prevent it from happening again.
GlucaGen will not work properly in patients:
- who have fasted for a longer period,
- with low adrenaline levels, adrenal insufficiency,
- with chronic hypoglycaemia (low blood sugar levels recurring for a longer period),
- with low blood sugar levels caused by alcohol consumption,
- with a diagnosed tumor secreting glucagon or insulin.
If any of the above situations apply to the patient, discuss it with your doctor or pharmacist.
GlucaGen and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
The following medicines may affect the action of GlucaGen 1 mg HypoKit:
- insulin - used to treat diabetes,
- indomethacin - used to treat pain and stiffness in the joints.
GlucaGen 1 mg HypoKit may affect the action of the following medicines:
- warfarin - used to prevent blood clots. GlucaGen may increase the anticoagulant effect of warfarin,
- beta-adrenergic blockers - used to treat high blood pressure and heart rhythm disorders. GlucaGen 1 mg HypoKit may temporarily increase blood pressure and cause an increased heart rate. In patients with coronary artery disease, if blood pressure or heart rate increases, treatment may be necessary.
Pregnancy, breastfeeding and fertility
Pregnancy, breastfeeding
If the patient is pregnant or breastfeeding, suspects that she may be pregnant or plans to have a child, GlucaGen 1 mg HypoKit may be used in case of severe hypoglycaemia.
If the patient is pregnant or breastfeeding, suspects that she may be pregnant or plans to have a child, she should consult her doctor or pharmacist before using any medicine.
Fertility
No data available.
Driving and using machines
After experiencing very low blood sugar levels, do not drive or operate machinery until the symptoms have resolved.
GlucaGen 1 mg HypoKit contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means it is considered 'sodium-free'.
3. How to use GlucaGen 1 mg HypoKit
This medicine should always be used as directed by your doctor. In case of doubts, consult your doctor.
Preparation and administration of the solution for injection
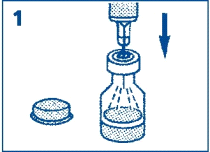
- 1. Remove the plastic cap from the vial. Remove the needle shield from the pre-filled syringe. Do not remove the plastic protective element located on the edge of the pre-filled syringe. Pierce the rubber stopper of the vial (through the marked circle) containing the glucagon powder, and then inject all the liquid from the pre-filled syringe into the vial.
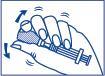
- 2. Without removing the needle, gently shake the vial until the powder is completely dissolved and the solution is clear.
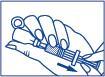
- 3. Check that the plunger is fully pressed. Holding the needle submerged in the liquid, slowly draw the entire solution from the vial back into the pre-filled syringe. Be careful not to pull the plunger out of the pre-filled syringe. Remove any air bubbles that may be present in the pre-filled syringe:
- holding the pre-filled syringe with the needle pointing upwards, gently tap it with your finger;
- by gently pressing the plunger, remove the air that has accumulated in the upper part of the pre-filled syringe.
Press the plunger until the correct dose is set.
When pressing the plunger, a small amount of liquid will be pushed out.
Read the information below about dosing.
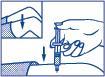
- 4. Inject the dose of the medicine subcutaneously or intramuscularly.
- 5. Place the unconscious person in a safe position, preventing choking.
- 6. Give the patient a carbohydrate-rich snack, such as sweets, cakes or fruit juice, as soon as the patient regains consciousness and is able to swallow. A carbohydrate-rich snack will help prevent another episode of low blood sugar.
After using GlucaGen 1 mg HypoKit, contact your doctor. Determine what caused the very low blood sugar level and how to prevent it from happening again.
Dosing
Therapeutic indications (severe hypoglycaemia)
The recommended dose is:
- Adults: administer 1 mg, which is the entire contents of the pre-filled syringe (1 ml) - marked on the pre-filled syringe as "1.0".
Children:
- children with a body weight below 25 kg or under 6-8 years of age: administer 0.5 mg, which is half the contents of the pre-filled syringe (0.5 ml) - marked on the pre-filled syringe as "0.5",
- children with a body weight above 25 kg or over 6-8 years of age: administer 1 mg, which is the entire contents of the pre-filled syringe (1 ml) - marked on the pre-filled syringe as "1.0".
Using a higher dose of GlucaGen than recommended
A higher dose of GlucaGen may cause nausea and vomiting. Treatment is usually not necessary. In the case of doses significantly higher than recommended, the serum potassium level may decrease, so it should be monitored.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur during treatment with this medicine:
Contact your doctor immediately if you experience any of the following serious side effects:
Very rare side effects(may occur less frequently than in 1 in 10,000 patients):
- allergic reaction - which may manifest as wheezing, sweating, rapid heartbeat, rash, facial swelling and collapse.
Other side effects
Common side effects(may occur less frequently than in 1 in 10 patients):
- feeling of nausea (nausea).
Uncommon side effects(may occur less frequently than in 1 in 100 patients):
- vomiting.
Rare side effects(may occur less frequently than in 1 in 1,000 patients):
- abdominal pain.
Frequency not known(cannot be estimated from the available data):
- reactions at the injection site.
If you experience any of the above side effects, as well as any side effects not listed in this leaflet, tell your doctor.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorisation holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store GlucaGen 1 mg HypoKit
- Store the medicine out of sight and reach of children.
- Do not freeze. Store in a refrigerator (2°C - 8°C). It is possible to store at a temperature below 25°C for 18 months, with the expiry date being maintained. Protect from light.
- The prepared medicine should be used immediately after preparation - do not store the solution for later use.
- Do not use this medicine after the expiry date stated on the label after: "EXP". The expiry date refers to the last day of the month stated.
- Do not use this medicine if the solution contains solid particles or the powder has not dissolved completely.
- Do not use the medicine if the plastic cap is loose or missing - in such cases, return the medicine to the pharmacy where it was dispensed.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What GlucaGen contains
- The active substance of the medicine is glucagon in the form of glucagon hydrochloride. One vial contains 1 mg of glucagon, which corresponds to 1 mg (1 IU) of glucagon in 1 ml of solution after preparation.
- The other ingredients are: lactose monohydrate, water for injections, hydrochloric acid (to adjust pH), sodium hydroxide (to adjust pH).
What GlucaGen looks like and contents of the pack
The GlucaGen 1 mg HypoKit pack contains a vial with a white or almost white powder containing 1 mg of glucagon and a pre-filled syringe with a clear, colourless and particle-free solvent.
Marketing authorisation holder and manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
For more information, contact your local representative of the marketing authorisation holder:
Novo Nordisk Pharma Sp. z o.o.
Tel.: 22 444 49 00
Fax: 22 444 49 01
Date of last revision of the leaflet:
Other sources of information
Detailed information about this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products: www.urpl.gov.pl
Information intended only for healthcare professionals
Read the entire leaflet before reading the additional information.
Identifiability
In order to improve the identifiability of biological medicinal products, the name and batch number of the administered product should be clearly recorded.
Due to the instability of GlucaGen in solution, the medicine should be administered immediately after preparation and should not be administered as an intravenous infusion.
Do not put the needle shield back on the used pre-filled syringe. Place the used pre-filled syringe in the orange outer packaging and, as soon as possible, transfer it to a sharps container.
Therapeutic indications in severe hypoglycaemia
Administer by subcutaneous or intramuscular injection.
If the patient does not respond within 10 minutes, administer glucose intravenously. If the patient has responded to treatment, administer carbohydrates orally to renew liver glycogen and prevent another episode of hypoglycaemia.
Adults:
Administer 1 mg by subcutaneous or intramuscular injection.
Children and adolescents (<18 years):< em>
Administer
- 0.5 mg (children with a body weight below 25 kg or under 6-8 years of age)
- 1 mg (children with a body weight above 25 kg or over 6-8 years of age).
Diagnostic indications (inhibition of gastrointestinal motility)
GlucaGen must be administered only by healthcare professionals during diagnostic procedures.
After the diagnostic procedure, administer carbohydrates orally to the patient if it is in line with the diagnostic procedure used. Note that GlucaGen has an action antagonistic to insulin.
GlucaGen may cause increased myocardial oxygen demand, increased arterial blood pressure and increased heart rate. Patients with heart disease who have been administered glucagon for diagnostic purposes should be monitored and treated if necessary.
GlucaGen used for diagnostic purposes in patients with diabetes may cause transient hyperglycaemia. In patients with diabetes, monitor blood glucose levels during treatment with the medicine and take appropriate treatment if necessary.
Note: A syringe with a thinner needle and a precise scale may be more suitable for diagnostic procedures.
Adults:
The dose is from 0.2 mg to 2 mg, depending on the diagnostic technique and route of administration.
In diagnostic procedures, the dose used to relax the stomach, duodenal bulb, duodenum and small intestine is from 0.2 mg to 0.5 mg by intravenous administration or 1 mg by intramuscular administration. The dose used to relax the colon is from 0.5 mg to 0.75 mg by intravenous administration or from 1 mg to 2 mg by intramuscular administration.
After intravenous administration of doses from 0.2 mg to 0.5 mg, the onset of action occurs within 1 minute and lasts from 5 to 20 minutes.
After intramuscular administration of doses from 1 mg to 2 mg, the onset of action occurs after 5 to 15 minutes and lasts for about 10 to 40 minutes.
Children and adolescents (<18 years):< em>
The safety and efficacy of the medicinal product GlucaGen in the inhibition of gastrointestinal motility during diagnostic tests have not been established in children and adolescents. There are no available data.
Additional side effects occurring after use in diagnostic procedures
Very rare(may occur less frequently than in 1 in 10,000 patients):
hypotension, hypertension, rapid heartbeat.
Uncommon(may occur less frequently than in 1 in 100 patients):
hypoglycaemia and hypoglycaemic coma.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterNovo Nordisk A/S
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Alternatives to Glucagen 1 mg Hipokit in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Glucagen 1 mg Hipokit in Ukraine
Alternative to Glucagen 1 mg Hipokit in Spain
Online doctors for Glucagen 1 mg Hipokit
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Glucagen 1 mg Hipokit – subject to medical assessment and local rules.










