
Gadovist 1,0

Ask a doctor about a prescription for Gadovist 1,0

How to use Gadovist 1,0
PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER
Gadovist 1.0, 1.0 mmol/ml, solution for injection
Gadobutrol
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult your doctor (radiologist) or the MRI department staff.
- If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor or the MRI department staff. See section 4.
Table of contents of the leaflet:
- 1. What is Gadovist 1.0 and what is it used for
- 2. Important information before using Gadovist 1.0
- 3. How to use Gadovist 1.0
- 4. Possible side effects
- 5. How to store Gadovist 1.0
- 6. Contents of the pack and other information
1. What is Gadovist 1.0 and what is it used for
This medicine is for diagnostic use only.
Gadovist 1.0 is a contrast agent for use in magnetic resonance imaging (MRI) to visualize various parts of the body, such as the brain, spine, head, neck, chest, breasts, abdomen (including pancreas, liver, and spleen), pelvis (including prostate, bladder, uterus), the retroperitoneal space (including kidneys), limbs (upper and lower) and the musculoskeletal system (muscles, bones, and joints), blood vessels, and heart. It can be used to assess myocardial perfusion under stress conditions (e.g., caused by medications). It can be used to diagnose myocardial viability (e.g., to detect the presence of scar tissue).
MRI is a type of diagnostic imaging in medicine that uses the different behavior of water molecules in healthy and diseased tissues. The examination is performed using complex magnet and radio wave systems. The activity is recorded by computers, which translate it into images.
Gadovist 1.0 is used in adults and children of all ages (including newborns).
Gadovist 1.0 is administered by injection into a vein by healthcare professionals. Gadovist 1.0 will be administered immediately before the MRI examination.
After injection, the patient will be monitored for at least 30 minutes.
The actual dose of Gadovist 1.0 suitable for a particular patient will depend on the patient's body weight and the area of the body being examined.
In adults, a single injection of a dose of 0.1 milliliter of Gadovist 1.0 per kilogram of body weight (meaning a 70 kg person will receive a dose of 7 milliliters) is recommended. In central nervous system (CNS) imaging and contrast-enhanced magnetic resonance angiography (CE-MRA), a maximum of 0.3 milliliter of Gadovist 1.0 per kilogram of body weight (meaning a 70 kg person will receive a dose of 21 milliliters) can be administered. In CNS imaging, the minimum dose that can be administered is 0.075 milliliter of Gadovist 1.0 per kilogram of body weight (meaning a 70 kg person will receive a dose of 5.25 milliliters).
For MRI imaging of the brain, spine, liver, and kidneys, a single injection of 0.1 milliliter of Gadovist 1.0 per kilogram of body weight is usually sufficient.
For MRI imaging of vessels, depending on the type of examination, a single injection of 7.5 to 15 milliliters (for patients weighing less than 75 kilograms) or 10 to 20 milliliters (for patients weighing 75 kilograms or more) is recommended.
Dosing in special patient groups
Gadovist 1.0 is not recommended for use in patients with severe kidney disease or in patients who have recently undergone or are scheduled to undergo liver transplantation. However, if the use of the medicine is necessary, the patient should receive only one dose of Gadovist 1.0 during the examination and should not receive a second injection for at least 7 days.
Use in children and adolescents
In children of all ages (including newborns), a single injection of a dose of 0.1 milliliter of Gadovist 1.0 per kilogram of body weight (see section 1: "What is Gadovist 1.0 and what is it used for") is recommended. Due to the immature kidney function in newborns (children up to 4 weeks of age) and infants (children up to 1 year of age), Gadovist 1.0 should only be used in these patients after careful consideration by the doctor. Newborns and infants should receive a single dose during the examination and should not receive a second injection for at least 7 days.
Elderly patients
No dose adjustment is necessary in patients over 65 years of age, but a blood test to check kidney function should be performed.
More information on the administration and preparation of Gadovist 1.0 can be found at the end of the leaflet.
Overdose of Gadovist 1.0:
To date, no cases of overdose have been reported. If this were to happen, the doctor would treat any symptoms that occur and check that the patient's heart and kidneys are working properly.
In case of any further doubts about the use of the medicine, you should consult your doctor or the MRI department staff.
4. Possible side effects
Like all medicines, Gadovist 1.0 can cause side effects, although not everybody gets them. Most of these reactions occur within half an hour of administering Gadovist 1.0.
In rare cases, delayed allergic reactions or other types of adverse reactions have been observed, occurring within several hours to several days after administering Gadovist 1.0. If this happens, you should immediately inform your doctor or radiologist.
Most side effects are mild to moderate. The most common side effects observed in patients receiving Gadovist 1.0 (which may occur in 5 or more out of 1,000 patients) are: headache, nausea (feeling sick), and dizziness.
The most serious side effects(in some cases life-threatening or fatal) are: cardiac arrest (heart stops beating), severe respiratory disease (acute respiratory distress syndrome) / fluid in the lungs (pulmonary edema) and severe allergic reactions (anaphylactoid reactions) (including respiratory arrest and anaphylactoid shock.
Additionally, adverse reactions have been observed (in some cases life-threatening or fatal): shortness of breath (shallow breathing) and loss of consciousness (fainting).
Rarely, allergic reactionsmay occur, including severe reactions that may require medical intervention.
The patient should immediately inform the MRI department staffif they experience:
- swelling of the face, lips, tongue, or throat,
- coughing and sneezing,
- difficulty breathing,
- itching,
- runny nose,
- hives (a specific type of rash).
These symptoms may be the first signs of a severe reaction, which means that it may be necessary to interrupt the examination and administer appropriate treatment.
The most common side effects(which may occur in 5 or more out of 1,000 patients) are: headache, nausea, dizziness.
Most side effects are mild to moderate.
Below are the possible side effects observed during clinical trials conducted before the marketing authorization of the medicine, by frequency of occurrence.
Frequent(may occur in up to 1 in 10 patients):
- headache
- nausea (feeling sick) Uncommon(may occur in up to 1 in 100 patients):
- allergic reactions (hypersensitivity, anaphylactoid reaction), e.g.,
- low blood pressure (hypotension)
- hives
- facial swelling (facial edema)
- eyelid swelling (periorbital edema)
- redness Frequency not known(cannot be estimated from the available data):
- anaphylactoid shock (severe allergic reaction)
- circulatory collapse (shock)
- respiratory arrest (respiratory failure)
- bronchospasm (difficulty breathing)
- cyanosis (blue discoloration of the lips)
- oral and pharyngeal edema (swelling of the mouth and throat)
- laryngeal edema (swelling of the larynx)
- high blood pressure
- chest pain
- angioedema (e.g., facial, throat, lip, or tongue swelling)
- conjunctivitis
- excessive sweating (increased sweating)
- coughing
- sneezing
- burning sensation
- pallor (pale skin)
- dizziness, taste disturbances (taste disorders), paresthesia (tingling and numbness)
- shortness of breath (shallow breathing)
- vomiting
- flushing (redness of the skin)
- itching (including generalized itching) (pruritus)
- rash (including generalized rash, maculopapular rash [small, flat, red spots], papular rash [small, raised, limited lesions], pruritic rash [itchy rash])
- various injection site reactions (e.g., leakage into surrounding tissues, burning sensation, cold sensation, warmth, redness, itching, pain, or bruising)
- feeling of heat Rare(may occur in up to 1 in 1,000 patients):
- loss of consciousness (fainting)
- seizures
- olfactory hallucinations (disturbed sense of smell)
- tachycardia (rapid heart rate)
- palpitations (heart pounding)
- dry mouth
- malaise
- feeling of cold
Adverse reactions reported after the marketing authorization of Gadovist 1.0
Frequency not known(cannot be estimated from the available data):
- cardiac arrest (heart stops beating)
- severe respiratory disease (acute respiratory distress syndrome)
- fluid in the lungs (pulmonary edema)
- reports of nephrogenic systemic fibrosis (a disease associated with skin hardening, which can also affect soft tissues and internal organs), most of which occurred in patients who received Gadovist 1.0 in combination with other gadolinium-containing medicines. After administering Gadovist 1.0, changes in kidney function tests (e.g., increased serum creatinine levels) have been observed.
Reporting of side effects
If you experience any side effects, including any not listed in this leaflet, you should inform your doctor or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Gadovist 1.0
Keep out of the sight and reach of children.
Store in a temperature not exceeding 30°C.
Chemical, physical, and microbiological stability has been demonstrated for 24 hours at a temperature between 20 and 25°C. From a microbiological point of view, the product should be used immediately after opening.
Do not use Gadovist 1.0 after the expiry date stated on the packaging after the expiry date (EXP). The expiry date refers to the last day of the month.
The batch number on the packaging is stated after the "Lot" label.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Gadovist 1.0 contains
- The active substance of the medicine is gadobutrol. 1 milliliter of the solution for injection contains 604.72 mg of gadobutrol (equivalent to 1.0 mmol of gadobutrol containing 157.25 mg of gadolinium).
- The other ingredients are: calcium dibekentate, trometamol, hydrochloric acid 3.6%, and water for injection. 1 vial contains 2 milliliters of the solution for injection, equivalent to 1209.44 mg of gadobutrol. 1 vial contains 7.5 milliliters of the solution for injection, equivalent to 4535 mg of gadobutrol. 1 vial contains 10 milliliters of the solution for injection, equivalent to 6047.20 mg of gadobutrol. 1 vial contains 15 milliliters of the solution for injection, equivalent to 9070 mg of gadobutrol. 1 vial contains 30 milliliters of the solution for injection, equivalent to 18141 mg of gadobutrol. 1 pre-filled syringe contains 5.0 milliliters of the solution for injection, equivalent to 3023 mg of gadobutrol. 1 pre-filled syringe contains 7.5 milliliters of the solution for injection, equivalent to 4535 mg of gadobutrol. 1 pre-filled syringe contains 10 milliliters of the solution for injection, equivalent to 6047 mg of gadobutrol. 1 pre-filled syringe contains 15 milliliters of the solution for injection, equivalent to 9070 mg of gadobutrol. 1 pre-filled syringe contains 20 milliliters of the solution for injection, equivalent to 12094 mg of gadobutrol. 1 syringe driver cartridge contains 15 milliliters of the solution for injection, equivalent to 9070 mg of gadobutrol. 1 syringe driver cartridge contains 20 milliliters of the solution for injection, equivalent to 12094 mg of gadobutrol. 1 syringe driver cartridge contains 30 milliliters of the solution for injection, equivalent to 18140 mg of gadobutrol. 1 infusion bottle contains 65 milliliters of the solution for injection, equivalent to 39307 mg of gadobutrol.
What Gadovist 1.0 looks like and contents of the pack
Before use, the medicine should be inspected. Gadovist 1.0 is a clear, colorless or pale yellow solution. Do not use Gadovist 1.0 if there is significant discoloration, particulate matter, or container damage.
Packaging contains:
1 or 3 vials, each containing 2 milliliters of the solution for injection.
1 or 10 vials, each containing 7.5, 15, or 30 milliliters of the solution for injection.
10 vials, each containing 10 milliliters of the solution for injection.
1 or 5 pre-filled syringes, each containing 5, 7.5, or 10 milliliters of the solution for injection (in a 10 milliliter glass or plastic syringe).
1 or 5 pre-filled syringes containing 15 milliliters of the solution for injection (in a 17 milliliter glass syringe or a 20 milliliter plastic syringe).
1 or 5 pre-filled syringes containing 20 milliliters of the solution for injection (in a 20 milliliter glass or plastic syringe).
1 or 5 syringe driver cartridges containing 15, 20, or 30 milliliters of the solution for injection (65 milliliter cartridge).
1 or 10 bottles containing 65 milliliters of the solution for injection (100 milliliter infusion bottle).
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Bayer AG
Kaiser-Wilhelm-Allee 1
51373 Leverkusen,
Germany
Manufacturer:
Bayer AG
Müllerstrasse 178
13353 Berlin
Germany
To obtain more detailed information, you should contact the representative of the marketing authorization holder:
Bayer Sp. z o.o.
Al. Jerozolimskie 158
02-326 Warsaw
Poland
phone: (0-22) 572 35 00
Date of last revision of the leaflet: July 2025
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only (see also sections 1 to 6):
Method of administration
The medicine is for intravenous injection only. After administration, the patient should be monitored for at least half an hour, as most adverse reactions occur during this period.
The required dose should be administered in a single rapid intravenous injection. The MRI examination with contrast agent can be started immediately after injection (or shortly after injection, depending on the pulse sequences and examination protocol used) due to the rapid onset of the contrast-enhancing effect. Optimal contrast enhancement occurs during the first arterial passage in the case of angiographic examination (contrast-enhanced magnetic resonance angiography - CE-MRA) and within about 15 minutes after administering Gadovist 1.0 in the case of central nervous system (CNS) imaging (time depends on the type of pathological change and tissue).
Instructions for use
- Before administration Before use, the medicine should be inspected. Do not use Gadovist 1.0 if there is significant discoloration, particulate matter, or container damage.
- Preparation of the dose Vials Gadovist 1.0 should be drawn up into a syringe immediately before use. The vial stopper should not be pierced more than once. Any unused medicine should be discarded after a single examination. Pre-filled syringes The Gadovist product is ready for use. The pre-filled syringe should be prepared for injection immediately before use. The tip cap of the pre-filled syringe should be removed immediately before use. Any unused medicine should be discarded after a single examination. Glass pre-filled syringes (only): MANUAL INJECTION
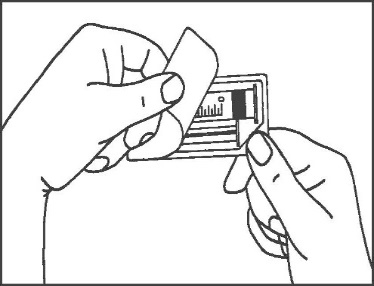
- 1. Open the packaging
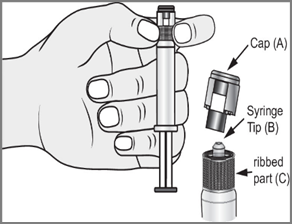
- 2.
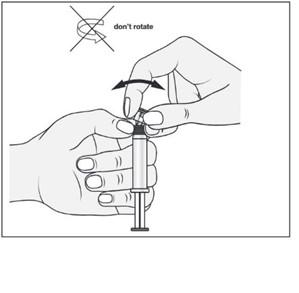
- 3. With the other hand, grasp the tip (A) of the closure system and gently tilt it back and forth until the tip detaches and can be removed (all seals will be broken)
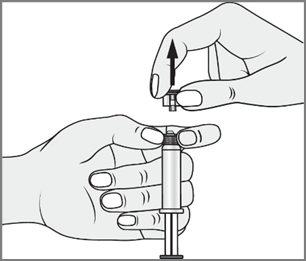
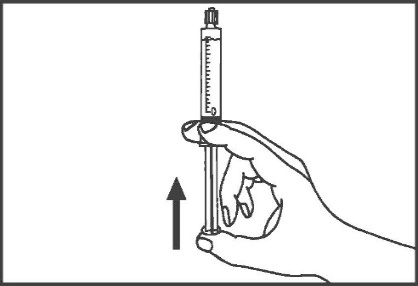
- 4. Remove the tip (A) in a straight upward direction. Do not touch the tip of the syringe (B) to maintain its sterility.
Plastic pre-filled syringes:
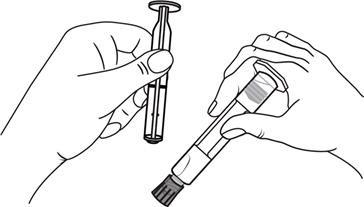
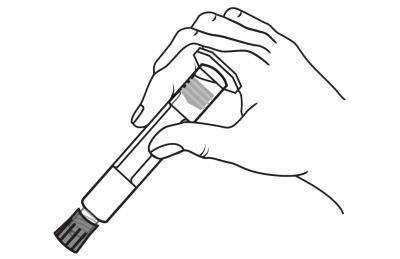
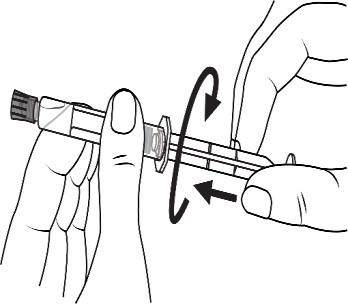
Open the tip cap by twisting
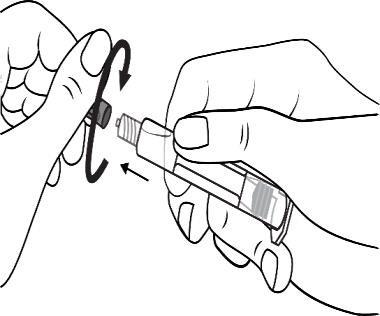
Screw the plunger into the syringe in a clockwise direction
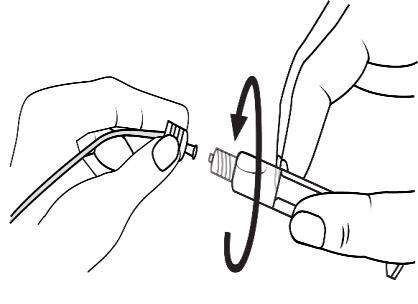
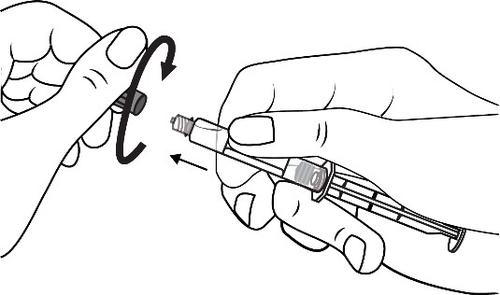
Connect the tip of the syringe to the cannula by rotating it in a clockwise direction. Then follow the instructions for use.
Open the tip cap by twisting
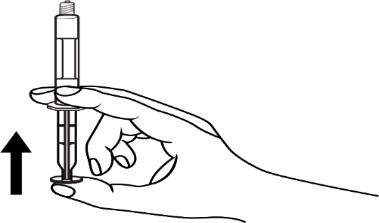
- 4. Remove air from the syringe
Syringe driver cartridges
The medicine should be administered by specialized medical personnel using appropriate procedures and equipment.
Sterility should be maintained during all injections of the medicine.
The instructions for use of the device should be provided by the manufacturer.
Any unused medicine should be discarded after a single examination.
Bottles
The bottle contains 65 milliliters of the solution for injection.
The medicine can be administered using an automatic dispenser.
The instructions for use of the device should be provided by the manufacturer.
Other information, see section: "How to store Gadovist 1.0".
Any unused medicine or waste material should be disposed of in accordance with local regulations.
The torn label from the vial or pre-filled syringe or cartridge or bottle should be attached to the patient's card to ensure proper registration of the administered gadolinium-containing medicine. The dose should also be recorded. If electronic patient cards are used, the name of the medicine, batch number, and dose should be entered in the patient's record.
Incompatibilities
As compatibility studies have not been conducted, the medicine should not be mixed with other medicinal products.
Dosing
The smallest dose that provides sufficient contrast enhancement for diagnostic purposes should be used. The dose should be calculated based on the patient's body weight and should not exceed the recommended dose per kilogram of body weight specified in this section.
Adults
The dose depends on the indication. A single injection of 0.1 mmol of Gadovist 1.0 per kilogram of body weight (which is 0.1 ml/kg body weight) is usually sufficient. The total maximum dose is 0.3 mmol of Gadovist 1.0 per kilogram of body weight (which is 0.3 ml/kg body weight).
CNS imaging:
Typically, a dose of 0.1 mmol per kilogram of body weight is recommended to make a diagnosis.
If, despite a negative MRI result, there is still a significant clinical suspicion of a pathological change or if more accurate information may affect patient treatment, a second injection of the medicine may be administered, adjusting the dose to a maximum of 0.2 mmol/kg body weight within 30 minutes of the first administration. In CNS imaging, the minimum dose that can be administered is 0.075 mmol of gadobutrol per kilogram of body weight (which is 0.075 ml of Gadovist 1.0 per kilogram of body weight).
Whole-body MRI imaging (excluding CE-MRA):
Typically, a dose of 0.1 ml/kg body weight of Gadovist 1.0 is sufficient to make a diagnosis.
Angiographic imaging:
Imaging of 1 field of view (FOV):
- 7.5 milliliters at a body weight below 75 kilograms; 10 milliliters at a body weight of 75 kilograms or above (which is 0.1-0.15 mmol/kg body weight)
- Imaging of more than 1 field of view (FOV):
- 15 milliliters at a body weight below 75 kilograms; 20 milliliters at a body weight of 75 kilograms or above (which is 0.2-0.3 mmol/kg body weight)
Special patient groups
Children and adolescents
In children of all ages (including newborns), the recommended dose is 0.1 mmol of gadobutrol per kilogram of body weight (see section 1: "What is Gadovist 1.0 and what is it used for"). Due to the immature kidney function in newborns (up to 4 weeks of age) and infants (up to 1 year of age), Gadovist 1.0 should only be used in these patients after careful consideration, at a dose not exceeding 0.1 mmol/kg body weight. During the examination, no more than one dose should be administered. Due to the lack of data on repeated administration, injections of Gadovist 1.0 should not be repeated unless the intervals between injections are at least 7 days.
Renal impairment
Gadovist 1.0 can be used in patients with severe renal impairment (glomerular filtration rate, GFR <30 ml min 1.73 m2) and in patients the perioperative period of liver transplantation only after careful consideration benefit-risk ratio if diagnostic information is essential not available for mri examination without contrast enhancement. use gadovist 1.0 necessary, dose should exceed 0.1 mmol kg body weight. no more than one be administered during imaging. due to lack data on repeated administration, injections unless intervals between are at least 7 days.< p>
Special warnings and precautions for use
Hypersensitivity
As with other contrast agents, the use of Gadovist 1.0 may be associated with the risk of anaphylactoid reactions, hypersensitivity, or idiosyncrasy, characterized by symptoms from the cardiovascular, respiratory, or skin systems, to severe reactions, including shock. Generally, patients with cardiovascular disease are more prone to severe symptoms, even death, due to severe hypersensitivity reactions.
The risk of hypersensitivity reactions may be increased in:
- patients with a history of reactions to contrast agents,
- patients with a history of asthma,
- patients with a history of allergic diseases.
In patients with a tendency to allergies, the decision to use Gadovist 1.0 must be made after careful consideration of the benefit-risk ratio. Most reactions occur within half an hour of administering the medicine. Therefore, it is recommended to monitor the patient after the examination. Access to medicines used in hypersensitivity reactions and appropriate equipment should be ensured. Rarely, delayed reactions (after several hours to several days) have been observed. Renal impairment
Before administering Gadovist 1.0, it is recommended to perform a renal function test in all patients.
Reports of nephrogenic systemic fibrosis (NSF) have been associated with the use of some gadolinium-containing medicines in patients with acute or chronic severe renal impairment (GFR <30 ml min 1.73 m2). patients undergoing liver transplantation are particularly at risk, as the risk of acute renal failure in this patient group is high. due to possibility nsf with use gadovist 1.0, medicine can be used severe impairment and perioperative period only after careful consideration benefit-risk ratio if diagnostic information essential not available for mri examination without contrast enhancement. 1.0 necessary, dose should exceed 0.1 mmol kg body weight. no more than one administered during imaging. lack data on repeated administration, injections unless intervals between least 7 days.
Since the renal clearance of gadobutrol may be reduced in elderly patients, especially in those over 65 years of age, it is particularly important to monitor patients for renal impairment.
Hemodialysis immediately after administering Gadovist 1.0 may facilitate its removal from the body. There is no evidence to justify the initiation of hemodialysis to prevent or treat NSF in patients not already undergoing hemodialysis.
Therefore, Gadovist 1.0 should be used in these patients after careful consideration of the benefit-risk ratio (see section 4.8).
Pregnancy and breastfeeding
Gadovist 1.0 should not be used during pregnancy unless the patient's clinical condition requires the use of gadobutrol.
The doctor, together with the breastfeeding mother, should decide whether to continue breastfeeding or to interrupt it for 24 hours after administering Gadovist 1.0.
Side effects
The summary of the safety profile is based on data from clinical trials involving over 6,300 patients and post-marketing surveillance.
Overdose
Single doses of gadobutrol up to 1.5 mmol/kg body weight have been well tolerated.
In case of overdose, as a precautionary measure, it is recommended to monitor cardiovascular function (including ECG) and check kidney function.
In case of overdose, Gadovist 1.0 can be removed from the body by dialysis.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBayer AG Bayer Schering Pharma AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Gadovist 1,0Dosage form: Solution, 279.3 mg/mlActive substance: gadobutrolManufacturer: BIPSO GmbH Bracco Imaging S.p.A.Prescription not requiredDosage form: Solution, 0.5 mmol/mlActive substance: gadoteric acidManufacturer: GE Healthcare AS GE Healthcare IrelandPrescription requiredDosage form: Solution, 0.5 mmol/mlActive substance: gadoteric acidManufacturer: GE Healthcare ASPrescription required
Alternatives to Gadovist 1,0 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Gadovist 1,0 in Україна
Alternative to Gadovist 1,0 in Іспанія
Online doctors for Gadovist 1,0
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Gadovist 1,0 – subject to medical assessment and local rules.














