
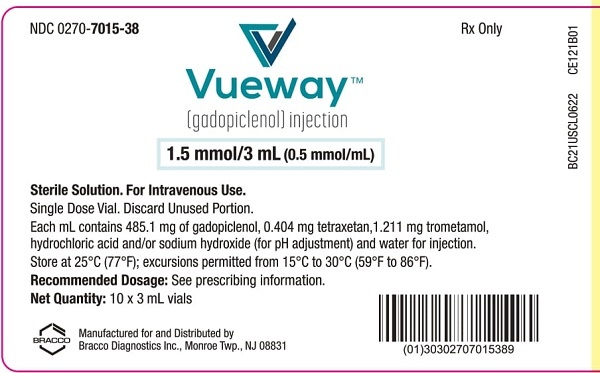
VUEWAY 0.5 mmol/mL Injectable Solution

How to use VUEWAY 0.5 mmol/mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Vueway 0.5 mmol/ml Solution for Injection
gadopiclenol
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, radiologist or pharmacist.
- If you get any side effects, talk to your doctor or radiologist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Vueway and what is it used for
- What you need to know before you are given Vueway
- How Vueway will be given to you
- Possible side effects
- Storage of Vueway
- Contents of the pack and other information
1. What is Vueway and what is it used for
Vueway is a contrast medium that enhances the contrast of images obtained during magnetic resonance imaging (MRI) examinations. Vueway contains the active substance gadopiclenol.
It improves the visualization and delineation of abnormal structures or lesions in certain parts of the body and helps to differentiate between healthy and diseased tissue.
It is used in adults and children (from 2 years of age).
It is administered by injection into a vein. This medicine is for diagnostic use only and will only be administered by healthcare professionals with experience in the clinical practice of magnetic resonance imaging.
2. What you need to know before you are given Vueway
Vueway must not be given
if you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor, radiologist or pharmacist before you are given Vueway:
- if you have had a previous reaction to any contrast agent,
- if you have asthma,
- if you have a history of allergy (such as hay fever, hives),
- if your kidneys do not work properly,
- if you have had seizures or are being treated for epilepsy,
- if you have any heart or blood vessel disease,
In all these cases, your doctor will decide whether the planned examination is possible or not. If you are given Vueway, your doctor or radiologist must take the necessary precautions and administer it under close supervision.
Your doctor or radiologist may decide to perform a blood test to check your kidney function before deciding to use Vueway, especially if you are 65 years of age or older.
Other medicines and Vueway
Tell your doctor, radiologist or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, tell your doctor, radiologist or pharmacist if you are receiving or have recently received medicines to treat heart or blood pressure conditions such as beta-blockers, vasoactive substances, angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists.
Pregnancy and breast-feeding
Pregnancy
Gadopiclenol may cross the placenta. It is not known if it affects the baby. Tell your doctor or radiologist if you are pregnant or think you may be pregnant, as Vueway should not be used during pregnancy unless it is strictly necessary.
Breast-feeding
Tell your doctor or radiologist if you are breast-feeding.
Your doctor will assess whether you should continue breast-feeding or interrupt it for up to 24 hours after receiving Vueway.
Driving and using machines
Vueway has a negligible influence on the ability to drive and use machines. However, if you feel unwell after the examination, you should not drive or use machines.
Vueway contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 15 ml vial; this is essentially "sodium-free".
3. How Vueway will be given to you
A healthcare professional will inject Vueway into your vein with a small needle. Vueway can be injected manually or with an automatic injector.
Your doctor or radiologist will determine the dose you should receive and will supervise the injection.
The usual dose of 0.1 ml/kg body weight is the same in adults and children from 2 years of age.
In children, your doctor or radiologist will use Vueway in vials with a single-use syringe to have greater precision of the injected volume.
After the injection, you will remain under supervision for at least 30 minutes. This is the time when most unwanted reactions (such as allergic reactions) may occur. However, in rare cases, reactions may occur after hours or days.
Use in patients with severe kidney problems
The use of Vueway is not recommended in patients with severe kidney problems. However, if its use is required, only a single dose should be administered during the examination and a second injection should not be administered until at least 7 days have passed.
Use in elderly patients
If you are 65 years of age or older, it is not necessary to adjust the dose, but you may have a blood test to check your kidney function.
If you receive more Vueway than you should
It is very unlikely that you will receive an overdose of Vueway, as it will be administered by a qualified healthcare professional. If this happens, Vueway can be removed from the body by haemodialysis (blood cleaning).
If you have any other questions about the use of this medicine, ask your doctor, radiologist or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
After the administration of Vueway, you will remain under observation. Most side effects occur within minutes. There is a small risk of having an allergic reaction to Vueway. These effects can occur immediately or up to seven days after the injection. These reactions can be severe and cause shock (a life-threatening allergic reaction).
Tell your doctor, radiologist or healthcare professional immediately if you experience any of the following side effects, as they may be the first signs of shock:
- swelling of the face, lips, tongue or throat
- dizziness (low blood pressure)
- difficulty breathing
- skin rash
- cough, sneezing or runny nose
The following side effects have been observed during clinical trials with Vueway and are listed according to their frequency:
Frequency | Possible side effects |
Common(may affect up to 1 in 10 people) | Injection site reaction* Headache |
Uncommon (may affect up to 1 in 100 people) | Allergic reaction** Diarrhoea Nausea (feeling sick) Fatigue (tiredness) Abdominal pain Unusual taste in the mouth Feeling of heat Vomiting (feeling sick) |
*The injection site reaction can be pain, swelling, feeling of cold or heat, bruising and redness.
**Allergic reactions can be: skin inflammation, skin redness, difficulty breathing, voice alteration, throat constriction, throat irritation, abnormal sensation in the mouth, transient facial redness (early reactions) and swollen eyes, swelling, skin rash and itching (late reactions).
Cases of nephrogenic systemic fibrosis (NSF) (which causes skin hardening and may also affect soft tissues and internal organs) have been reported with other gadolinium-containing contrast agents, but no cases of NSF have been reported with Vueway during clinical trials.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System: www.notificaRAM.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vueway
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial label or on the carton after "EXP" or "CAD". The expiry date refers to the last day of that month.
This medicine is a clear, colourless to pale yellow solution.
Do not use this medicine if the solution is not clear or if it contains visible particles.
For vials: This medicine does not require any special storage conditions.
Chemical and physical stability has been demonstrated for 24 hours at a temperature of up to 25°C.
From a microbiological point of view, the product should be used immediately after opening.
For pre-filled syringes: Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Vueway contains
- The active substance is gadopiclenol. Each ml of solution contains 485.1 mg of gadopiclenol (equivalent to 0.5 mmol of gadopiclenol and 78.6 mg of gadolinium).
- The other ingredients are tetraxetan, trometamol, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment) and water for injections. See section 2 "Vueway contains sodium".
Appearance and pack of Vueway
It is a clear, colourless to pale yellow solution for injection.
It is available in packs that include:
- 1 vial with 3, 7.5, 10, 15, 30, 50 or 100 ml of solution for injection.
- 25 vials with 7.5, 10 or 15 ml of solution for injection.
- 1 or 10 (10 x 1) pre-filled syringes with 7.5, 10 or 15 ml of solution for injection.
- 1 pre-filled syringe with 7.5, 10 or 15 ml of solution for injection with administration kit for manual injection (one extension line and one cannula).
- 1 pre-filled syringe with 7.5, 10 or 15 ml of solution for injection with administration kit for Optistar Elite injector (one extension line, one cannula and one empty 60 ml plastic syringe).
- 1 pre-filled syringe with 7.5, 10 or 15 ml of solution for injection with administration kit for Medrad Spectris Solaris EP injector (one extension line, one cannula and one empty 115 ml plastic syringe).
Not all pack sizes may be marketed.
Marketing authorisation holder
Bracco Imaging SPA
Via Egidio Folli, 50
20134 Milano
Italy
Manufacturer
Guerbet
16 rue Jean Chaptal
93600 Aulnay-sous-Bois
France
BIPSO GmbH
Robert-Gerwig-Strasse 4
Singen (Hohentwiel)
78224
Germany
Date of last revision of this leaflet December 2024
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
For more details on how to use the medicine, see section 6.6 Special precautions for disposal and other handling of the Summary of Product Characteristics of this medicine.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VUEWAY 0.5 mmol/mL Injectable SolutionDosage form: INJECTABLE, 0.5 mmol/mlActive substance: gadopiclenolManufacturer: GuerbetPrescription requiredDosage form: INJECTABLE, 0.5 mmol/mlActive substance: gadopiclenolManufacturer: GuerbetPrescription requiredDosage form: INJECTABLE, 0.5 mmol/mlActive substance: gadoteric acidManufacturer: Ge Healthcare Bio-Sciences, S.A.U.Prescription required
Online doctors for VUEWAY 0.5 mmol/mL Injectable Solution
Discuss questions about VUEWAY 0.5 mmol/mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















