
Furosemid Laboraturios Basi
Ask a doctor about a prescription for Furosemid Laboraturios Basi

How to use Furosemid Laboraturios Basi
Leaflet attached to the packaging: Information for the user
Furosemid Laboratórios Basi, 10 mg/mL, solution for injection/infusion
Furosemide
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What Furosemid Laboratórios Basi is and what it is used for
- 2. Important information before taking Furosemid Laboratórios Basi
- 3. How to take Furosemid Laboratórios Basi
- 4. Possible side effects
- 5. How to store Furosemid Laboratórios Basi
- 6. Contents of the pack and other information
1. What Furosemid Laboratórios Basi is and what it is used for
Furosemid Laboratórios Basi 10 mg/mL solution for injection/infusion contains the active substance furosemide. Furosemide belongs to a group of medicines called diuretics. Furosemid Laboratórios Basi is used in:
- 1. Treatment of edema (edema caused by excess fluid in the body), occurring in patients with:
- liver disease
- heart disease (e.g., pulmonary edema)
- kidney disease
- 2. Treatment of severely high blood pressure (hypertensive crisis).
Furosemid Laboratórios Basi is indicated for use in adults and adolescents over 15 years of age. The medicine can be used in infants and children under 15 years of age only in exceptional cases. How Furosemid Laboratórios Basi solution for injection/infusion works
- The Furosemid Laboratórios Basi solution for injection/infusion helps to remove more water (urine) than usual. If excess water in the body is not removed, it can lead to excessive strain on the heart, blood vessels, lungs, kidneys, or liver.
2. Important information before taking Furosemid Laboratórios Basi
Do not take Furosemid Laboratórios Basi
- if you are allergic to furosemide or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to sulfonamide antibiotics, such as sulfadiazine and cotrimoxazole;
- if you have kidney problems. In some types of kidney failure, this medicine can still be given. Your doctor will be able to determine if you can take this medicine;
- if you do not produce urine;
- if you have severe liver disease;
- if you have very low levels of potassium or sodium in your blood (confirmed by blood tests);
- if you have decreased blood volume (hypovolemia) or dehydration;
- if you are breastfeeding (see "Pregnancy and breastfeeding").
Warnings and precautions
Before taking Furosemid Laboratórios Basi, discuss with your doctor or nurse if you have:
- low blood pressure;
- diabetes;
- gout (pain associated with inflammation of the joints) caused by high levels of uric acid in the blood;
- obstruction of the urinary tract (e.g., enlarged prostate, kidney swelling due to urine accumulation, narrowing of the ureter);
- very low levels of protein in the blood, e.g., in nephrotic syndrome;
- liver disease;
- rapidly progressive kidney dysfunction associated with severe liver disease (e.g., liver cirrhosis);
- risk of severe arterial hypertension (e.g., if you have circulatory disorders in cerebral or coronary arteries);
- inflammatory disease called "systemic lupus erythematosus (SLE)";
- elderly patients who are also taking other medicines that may cause low blood pressure, or if you have other conditions associated with a risk of low blood pressure.
Especially during long-term treatment, your doctor may regularly check the levels of potassium, sodium, calcium, bicarbonate, creatinine, urea, uric acid, and glucose in your blood. Weight loss due to loss of body fluids should not exceed 1 kg of body weight per day.
Children
In the case of administration to premature infants, furosemide may cause kidney stones or calcifications. In premature infants, the duct between the pulmonary artery and the aorta, which is open in the unborn child, may remain open.
Furosemid Laboratórios Basi with other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take. This is important because some medicines should not be taken with furosemide or may require adjustment of the furosemide dose or the dose of the other medicine taken at the same time. In particular, tell your doctor or nurse if you are taking:
- lithium (used to treat mood disorders);
- cardiac glycosides (e.g., digoxin);
- terfenadine (used to treat allergies);
- levothyroxine (used to treat hypothyroidism);
- medicines used to treat high blood pressure, including thiazide diuretics (e.g., bendroflumethiazide or hydrochlorothiazide), ACE inhibitors (e.g., lisinopril), and angiotensin II antagonists (e.g., losartan);
- medicines used to treat diabetes (e.g., metformin and insulin);
- anti-inflammatory medicines, including NSAIDs (used to treat pain and inflammation, such as diclofenac, ibuprofen, indomethacin, and celecoxib) and acetylsalicylic acid in high doses (aspirin);
- corticosteroids (medicines used to treat inflammation or allergies, e.g., prednisolone, dexamethasone);
- carbenoxolone (used to treat stomach ulcers);
- laxatives;
- chloral hydrate (used to treat sleep problems);
- phenytoin (used to treat epilepsy);
- theophylline (used to treat asthma);
- probenecid (used to treat gout);
- methotrexate (used to treat certain cancers or severe arthritis);
- cyclosporin (prevents transplant rejection);
- medicines that increase blood pressure (vasopressors, such as adrenaline, noradrenaline), as they may not work properly when taken with furosemide;
- antibiotics (such as cephalosporins, aminoglycosides, polymyxins, and quinolones). The effect of aminoglycoside antibiotics (e.g., kanamycin, gentamicin, and tobramycin) on hearing may be increased by furosemide, especially in patients with kidney problems;
- foscarnet (used to treat diseases caused by single-celled organisms known as protozoa);
- iodinated contrast agents;
- cisplatin (used in anticancer chemotherapy);
- risperidone (used to treat mental disorders).
Furosemid Laboratórios Basi with food
Large amounts of licorice in combination with furosemide may lead to increased potassium loss.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor for advice before taking this medicine. Furosemide should not be used during pregnancy unless clearly necessary, as furosemide crosses the placental barrier. Administration of furosemide during pregnancy may lead to increased bilirubin levels in the fetus, resulting in jaundice and brain damage in the child. This medicine may also lead to increased urine production in the fetus. Furosemide passes into breast milk. Do not breastfeed while taking furosemide.
Driving and using machines
This medicine may affect your ability to react to such an extent that your ability to drive a car, operate machinery, or perform hazardous tasks may be impaired. This is especially true at the beginning of treatment, when increasing the dose, or when changing medicines, as well as when consuming alcohol.
Furosemid Laboratórios Basi contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per ampoule, i.e., the medicine is considered "sodium-free".
3. How to take Furosemid Laboratórios Basi
Your doctor will decide how much medicine you need, when it should be given, and how long the treatment will last. This will depend on your age, weight, medical history, other medicines you are taking, and the nature and severity of your disease. The lowest effective dose will always be used. Furosemide injection is usually given by a doctor or nurse:
- as a slow injection into a vein or
- exceptionally into a muscle.
In some cases, instead of injections, your doctor may recommend administering this medicine as a continuous intravenous infusion (drip). As soon as the patient's condition allows, oral administration will be initiated. AdultsIn the treatment of fluid retention in tissues (edema) and (or) fluid accumulation in the abdominal cavity (ascites) due to heart or liver disease, edema caused by kidney disease or pulmonary edema, the initial dose is 20 mg to 40 mg of furosemide. Then the dose will be gradually increased to a maximum dose of 1500 mg per day. In the treatment of severely high blood pressure, a single dose is 20 mg to 40 mg of furosemide. In adults, the maximum daily dose of furosemide should not exceed 1500 mg. ElderlyThe usual initial dose in elderly patients is 20 mg per day. Adults with renal impairmentIn patients with severe renal impairment (serum creatinine >5 mg/dL), it is recommended not to exceed an infusion rate of 2.5 mg of furosemide per minute.
Use in children
The average dose usually used in children is 0.5 mg of furosemide per kilogram of body weight per day. Exceptionally, up to 1 mg of furosemide per kilogram of body weight per day can be given.
Overdose of Furosemid
If you think you have taken too much of this medicine, tell your doctor immediately. Symptoms of acute or chronic overdose depend on the degree of loss of salts and fluids. Overdose symptoms include dry mouth, increased thirst, irregular heartbeat, mood changes, muscle cramps or pain, nausea or vomiting, unusual tiredness or weakness, and loss of appetite. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. In patients treated with furosemide, low blood pressure with dizziness, fainting, or loss of consciousness may occur. If you notice any of the following side effects, contact your doctor or nurse immediately:
- Severe allergic reaction, which can cause skin rash, facial swelling, lip, tongue, or throat swelling, difficulty breathing, and loss of consciousness (anaphylactic or anaphylactoid reaction) (may occur in less than 1 in 1,000 people);
- Stevens-Johnson syndrome, toxic epidermal necrolysis; their symptoms are initially reddish, target-like patches or round patches, often with central blisters on the torso. They can also cause mouth ulcers, throat, nose, genital, and eye ulcers (red and swollen eyes). These severe skin rashes can be preceded by fever and flu-like symptoms. Rashes can lead to widespread skin peeling and life-threatening complications or can lead to death (unknown).
- Rhabdomyolysis: a rare disease characterized by pain, tenderness, and weakness of the muscles; such muscle damage is often accompanied by severe potassium deficiency (unknown).
Other side effects
Very common(may affect more than 1 in 10 people)
- Electrolyte disturbances (including symptomatic), dehydration, and decreased circulating blood volume, especially in the elderly (symptoms such as increased thirst, headache, dizziness, fainting, disorientation, pain or weakness in joints or muscles, cramps or spasms, nausea or vomiting, rapid or irregular heartbeat); elevated levels of certain fats in the blood (triglycerides)
- Low blood pressure, including circulatory disorders when changing position from lying down to standing (when given intravenously)
- Elevated creatinine levels in the blood
Common(may affect less than 1 in 10 people)
- Concentration of blood (in case of excessive urine excretion)
- Decreased sodium and chloride levels in the blood, low potassium levels in the blood, elevated cholesterol levels in the blood, elevated uric acid levels in the blood, and worsening of gout
- Brain function disorders in patients with severe liver dysfunction (hepatic encephalopathy)
- Increased urine volume
Uncommon(may affect less than 1 in 100 people)
- Low platelet count (thrombocytopenia)
- Altered glucose tolerance. This may lead to worsening of the metabolic state in patients with existing diabetes (apparent diabetes). It may reveal previously undiagnosed diabetes (latent diabetes).
- Hearing disorders, although these are usually transient, especially in patients with kidney problems or low protein levels in the blood (e.g., in nephrotic syndrome) and (or) if the intravenous medicine is injected too quickly into a vein
- Deafness (sometimes irreversible)
- Malaise
- Itching, hives, rash, skin
- Mucous membrane reactions with redness, blistering, or peeling (e.g., bullous pemphigoid, erythema multiforme, pemphigus, exfoliative dermatitis, lichenoid dermatitis)
- Increased sensitivity of the skin to sunlight
Rare(may affect less than 1 in 1,000 people)
- Increased count of a certain type of white blood cell (eosinophilia)
- Decreased count of white blood cells (leukopenia)
- Tingling, numbness, or painful burning of limbs
- Ringing in the ears (tinnitus)
- Vasculitis
- Vomiting, diarrhea
- Kidney damage (interstitial nephritis)
- Fever
Very rare(may affect less than 1 in 10,000 people)
- Anemia caused by abnormal breakdown of red blood cells (hemolytic anemia)
- A condition in which the bone marrow stops producing enough new blood cells (aplastic anemia)
- Significant decrease in the number of certain types of white blood cells (agranulocytosis). Symptoms may include fever with chills, changes in the mucous membranes, and throat pain
- Acute pancreatitis
- Liver disorder called "intrahepatic cholestasis"
- Elevated liver enzyme activity in the blood, which can cause jaundice (yellowing of the skin, dark urine, fatigue)
Unknown(frequency cannot be estimated from the available data)
- Systemic lupus erythematosus (SLE) may worsen or be activated.
- Low calcium levels in the blood, low magnesium levels in the blood, decreased blood pH (metabolic acidosis), pseudo-Bartter syndrome (kidney failure associated with improper and (or) prolonged use of furosemide).
Common symptoms of low sodium levels in the blood are apathy, calf cramps, loss of appetite, weakness, drowsiness, vomiting, and disorientation. Low potassium levels in the blood may cause muscle weakness, sensory disturbances in the limbs (tingling, numbness, or painful burning), inability to move part of the body (paresis), gastrointestinal symptoms (vomiting, constipation, excessive gas in the digestive tract), kidney symptoms (excessive urine production, unnatural thirst), and heart symptoms (slow or irregular heartbeat). Significant potassium loss can lead to intestinal paralysis (paralytic ileus) or impaired consciousness, and even coma. Low calcium levels in the blood may rarely lead to tetany. Low magnesium levels in the blood may rarely cause tetany or cardiac arrhythmias.
- Dizziness, fainting, and loss of consciousness, headache
- Thrombosis, especially in elderly patients
- Excessive urine production, especially in elderly patients and children
- Circulatory problems (up to circulatory collapse) may occur, manifested mainly by headache, dizziness, blurred vision, dry mouth, and thirst, low blood pressure, and circulatory disorders when changing position from lying down to standing.
- Severe skin reactions (which may also affect the mucous membranes), such as acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS), and lichenoid reactions, which are characterized by small, itchy, reddish-purple spots on the skin, genitals, or in the mouth.
- Elevated sodium levels in urine, elevated chloride levels in urine, elevated urea levels in blood, symptoms of urinary tract obstruction, and even urinary retention;
- Calcification in the kidneys and (or) kidney stones in premature infants, kidney failure
- Increased risk of persistent ductus arteriosus in premature infants treated with furosemide in the first weeks of life
- Pain after injection into a muscle
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, PL-02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Furosemid Laboratórios Basi
Keep the medicine out of the sight and reach of children. Do not store above 25°C. Store in the original packaging to protect from light. Do not use this medicine after the expiry date stated on the carton: "Expiry date EXP". The expiry date refers to the last day of the month stated. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Furosemid Laboratórios Basi contains
- The active substance of the medicine is furosemide. Each 1 mL of solution contains 10 mg of furosemide. Each 2 mL ampoule contains 20 mg of furosemide.
- The other ingredients are sodium chloride, sodium hydroxide (to adjust pH), and water for injections.
What Furosemid Laboratórios Basi looks like and contents of the pack
Clear and colorless solution, free from visible particles. Orange glass type I ampoule with OPC point, containing 2 mL of solution for injection/infusion, in a cardboard box. Pack sizes: 50 ampoules
Marketing authorization holder
Laboratórios Basi – Indústria Farmacêutica, S.A., Parque Industrial Manuel Lourenço Ferreira, Lote 15, 3450-232 Mortágua, Portugal, Tel.: +351 231 920 250, Fax: +351 231 921 055, e-mail: [email protected]
Manufacturer
Laboratórios Basi – Indústria Farmacêutica SA, Parque Industrial Manuel Lourenço Ferreira Lotes 8, 15 e 16, 3450-232 Mortágua, Portugal
This medicine is authorized in the Member States of the European Economic Area under the following names:
Portugal: Furosemida Basi, Czech Republic: Furosemid Basi, Poland: Furosemid Laboratórios Basi, Bulgaria: Фуроземид Basi 10 mg//mL Инжекционен/инфузионен разтвор, Lithuania: Furosemide Basi 10 mg//mL Injekcinis ar infuzinis tirpalas, Romania: Furosemid Basi 10 mg//mL soluţie injectabilă/perfuzabilă, Sweden: Furosemide Basi 10 mg//mL Injektions-/infusionsvätska, lösning, Finland: Furosemide Basi 10 mg//mL Injektio-/infuusioneste, liuos, Denmark: Furosemid Basi, Norway: Furosemide Basi 10 mg//mL Injeksjons-/infusjonsvæske, oppløsning, Germany: Furosemid Basi 10 mg//mL Injektions-/Infusionslösung, Estonia: Furosemide Basi, Latvia: Furosemide Basi 10 mg//mL šķīdums injekcijām/infūzijām
Date of last revision of the leaflet:
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Incompatibilities
Solutions for injection/infusion with an acidic or slightly acidic pH and significant buffer capacity should not be mixed with Furosemid Laboratórios Basi 10 mg/mL solution for injection/infusion. In the case of these mixtures, the pH value is shifted to the acidic range, and the slightly soluble furosemide precipitates as a crystalline sediment. Furosemid Laboratórios Basi 10 mg//mL solution for injection/infusion should not be administered with other medicinal products in the same syringe. Instructions for dilution of the medicinal product prior to administration, see section "Instructions for disposal and preparation of the medicinal product for use". In nephrotic syndrome, the dose should be adjusted with caution due to the risk of increased side effects.
Shelf life
After first opening: The product has shown physical and chemical stability for 24 hours at room temperature. From a microbiological point of view, the product should be used immediately. If not used immediately, the user is responsible for the shelf life and storage conditions prior to use. Shelf life after dilution: Chemical and physical stability has been demonstrated for 24 hours at 25 °C. From a microbiological point of view, the product should be used immediately. If not used immediately, the user is responsible for the shelf life and storage conditions during use.
Instructions for disposal and preparation of the medicinal product for use
For single use only. The medicinal product should be used immediately after opening the ampoule. The remaining contents should be discarded after use. The medicinal product should be inspected before use. Do not use the medicinal product if signs of deterioration are visible (e.g., particles or discoloration). It can be diluted with:
- sodium chloride 9 mg//mL (0.9%) solution for injection,
- Ringer's solution,
- Ringer's solution with lactate.
Attention should be paid to ensuring that the pH of the solution used is slightly alkaline to neutral (pH not less than 7). Acidic solutions should not be used, as this may cause the active substance to precipitate (see section "Incompatibilities").
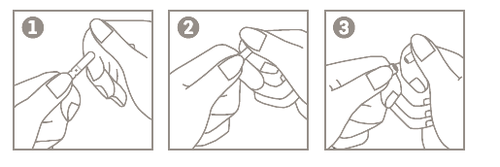
Instructions for opening ampoules (with a score line)
- 1. Hold the ampoule body between the thumb and index finger, with the tip facing upwards;
- 2. Place the index finger of the other hand on the top part of the ampoule, supporting it. Place your thumb over the score line;
- 3. By bringing the index fingers together, press the area marked with the score line to open the ampoule.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratórios Basi – Indústria Farmaceutica, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Furosemid Laboraturios BasiDosage form: Solution, 10 mg/mlActive substance: furosemidePrescription not requiredDosage form: Solution, 10 mg/mlActive substance: furosemidePrescription requiredDosage form: Tablets, 40 mgActive substance: furosemidePrescription required
Alternatives to Furosemid Laboraturios Basi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Furosemid Laboraturios Basi in Spain
Alternative to Furosemid Laboraturios Basi in Ukraine
Online doctors for Furosemid Laboraturios Basi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Furosemid Laboraturios Basi – subject to medical assessment and local rules.










