

Fulvestrant Sun

Ask a doctor about a prescription for Fulvestrant Sun

How to use Fulvestrant Sun
1. What is Fulvestrant SUN and what is it used for
This medicine contains the active substance fulvestrant, which blocks the action of estrogen receptors.
Estrogens, female sex hormones, may have an effect on the development of breast cancer.
Fulvestrant SUN is used:
- as the only medicine in the treatment of breast cancer (with estrogen receptors present), which is locally advanced or has spread to other parts of the body (metastases) in postmenopausal women, or
- in combination with palbociclib in the treatment of breast cancer with hormone receptors present, without excessive expression of the human epidermal growth factor receptor 2. These medicines are used when the tumor is locally advanced or has spread to other parts of the body (metastases). Pre-menopausal women will also receive a medicine called a luteinizing hormone-releasing hormone (LHRH) agonist.
When Fulvestrant SUN is given in combination with palbociclib, it is important to read the package leaflets of both medicines. If you have any questions about palbociclib, ask your doctor.
2. Important information before using Fulvestrant SUN
When NOT to use Fulvestrant SUN
- if you are allergic to fulvestrant or any of the other ingredients of this medicine (listed in section 6);
- if you are pregnant or breastfeeding;
- if you have severe liver function disorders.
Warnings and precautions
Inform your doctor, pharmacist, or nurse before using Fulvestrant SUN if you have ever had:
- kidney or liver disease;
- reduced platelet count (which enables blood to clot), or bleeding disorders;
- blood clotting disorders;
- osteoporosis (bone mass loss);
- alcohol dependence.
Children and adolescents
This medicine is not recommended for use in children and adolescents under 18 years of age.
Fulvestrant SUN and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
In particular, inform your doctor if you are taking anticoagulant medicines (medicines that prevent blood clots).
Pregnancy and breastfeeding
Fulvestrant SUN should not be used during pregnancy. If you may become pregnant, you should use effective contraception during treatment with Fulvestrant SUN and for 2 years after the last dose.
Do not breastfeed during treatment.
Driving and using machines
Fulvestrant SUN has not been shown to affect the ability to drive or use machines. If you feel tired after using this medicine, do not drive or use machines.
Fulvestrant SUN contains 10% v/v ethanol (alcohol),i.e. up to 1 g per dose, which is equivalent to 20 mL of beer or 8 mL of wine per dose. It is harmful to individuals with alcohol dependence.
This should be taken into account when using in pregnant or breastfeeding women and individuals at high risk, such as patients with liver disease or epilepsy.
Fulvestrant SUN containspurified castor oil, which may cause severe allergic reactions.
Fulvestrant SUN contains benzyl alcohol
This medicine contains 1 g of benzyl alcohol, which is equivalent to 100 mg/mL.
Benzyl alcohol may cause allergic reactions.
Pregnant or breastfeeding women and patients with liver or kidney disease should consult their doctor before using the medicine, as large amounts of benzyl alcohol may accumulate in the body and cause side effects (so-called metabolic acidosis)
3. How to use Fulvestrant SUN
Fulvestrant SUN is given by a doctor or nurse in two slow intramuscular injections, each into a different buttock.
The recommended dose is 500 mg of fulvestrant (two injections of 250 mg) given once a month, and an additional 500 mg given 2 weeks after the first dose.
If you have any further questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Severe side effects
In case of the following side effects, contact your doctor immediately:
- allergic reactions (including anaphylaxis), including swelling of the face, lips, tongue, and/or throat (common side effects), which may be symptoms of anaphylactic reactions;
- thromboembolic disease (increased risk of blood clots) * (common side effect). Symptoms may include pain, deep pain, and swelling in a specific area (especially in one leg), shortness of breath, and chest pain (if the clot moves to the lungs).
- hepatitis (uncommon side effect). Symptoms may include nausea, diarrhea, jaundice (yellowing of the skin or eyes), dark urine, pale stools, easy bruising, itching, or chills.
- liver failure (uncommon side effect). Symptoms may include nausea, diarrhea, jaundice (yellowing of the skin or eyes).
Other side effects
If you experience any of the following side effects, inform your doctor, pharmacist, or nurse
Very common side effects (occurring in more than 1 in 10 people):
- injection site reactions, such as pain and/or inflammation;
- changes in liver enzyme activity (in blood tests)*;
- nausea;
- feeling weak, tired*;
- joint and muscle pain;
- hot flashes;
- rash.
Common side effects (occurring in less than 1 in 10 people):
- headache;
- vomiting, diarrhea, or loss of appetite*;
- urinary tract infections;
- back pain*;
- increased bilirubin levels (a pigment produced by the liver);
- decreased platelet count;
- vaginal bleeding;
- lower back pain radiating to the leg (sciatica);
- sudden weakness, numbness, tingling, or loss of ability to move the leg, especially if it occurs only on one side of the body, sudden difficulty walking or maintaining balance (peripheral neuropathy).
Uncommon side effects (occurring in less than 1 in 100 people)
- thick, white vaginal discharge and vaginal yeast infection (infection);
- bruising and bleeding at the injection site;
- increased activity of gamma-glutamyltransferase, a liver enzyme measured in blood tests;
- numbness, tingling, and pain;
- anaphylactic reactions.
* Includes side effects for which the impact of Fulvestrant SUN cannot be assessed due to the underlying disease.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Fulvestrant SUN
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date (EXP) stated on the carton and blister.
The expiry date refers to the last day of the month.
Store and transport at a refrigerated temperature (2°C - 8°C).
Store the prefilled syringe in its original packaging to protect it from light.
Limit storage of the product at a temperature other than 2°C - 8°C. Avoid storage at temperatures above 30°C and do not exceed 28 days with an average storage temperature below 25°C (but above 2°C - 8°C).
If the temperature range is exceeded, apply the recommended storage conditions immediately (store and transport at a refrigerated temperature (2°C - 8°C)). Exceeding the recommended storage temperature may have a cumulative effect on the quality of the product and should not exceed 28 days during the shelf life. Exposure to temperatures below 2°C does not harm the product, provided it is not stored below -20°C.
Store the prefilled syringe in its original packaging to protect it from light.
Medical personnel are responsible for proper storage, use, and disposal of the packaging after using Fulvestrant SUN.
This medicine may pose a risk to aquatic environments. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Fulvestrant SUN contains
- The active substance is fulvestrant. One prefilled syringe (5 mL) contains 250 mg of fulvestrant.
- The other ingredients are: ethanol (96%), benzyl alcohol (E 1519), benzyl benzoate, and purified castor oil.
What Fulvestrant SUN looks like and contents of the pack
A clear, colorless to yellowish, viscous solution in a prefilled syringe equipped with a needle shield system, containing 5 mL of solution for injection.
To administer the recommended monthly dose of 500 mg, inject the contents of two prefilled syringes.
Fulvestrant SUN is available in 4 types of packaging, containing 1, 2, 4, or 6 prefilled syringes, and corresponding to 1, 2, 4, or 6 needles with a safety system. Not all pack sizes are marketed.
Marketing authorization holder/Manufacturer/Importer
Sun Pharmaceutical Industries Europe B.V., Polarisavenue 87, 2132 JH Hoofddorp, Netherlands
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Denmark, Germany, France, Italy, Netherlands, Norway, Romania, Spain, Sweden, United Kingdom: Fulvestrant SUN
Date of last revision of the leaflet: 17.12.2020
Information intended for healthcare professionals only
Fulvestrant SUN should be administered using two prefilled syringes (see section 3).
Administration instructions
Caution – do not sterilize the needle shield (BD SafetyGlide Shielding Hypodermic Needle) in an autoclave. When using and disposing of the needle, keep your hands behind the needle at all times.
Regarding the syringes:
- Remove the glass body of the prefilled syringe from the container and check if it is not damaged.
- Open the outer packaging of the needles.
- Before administering the solution, inspect it for the presence of solid particles and color changes.
- Hold the syringe vertically by the ribbed part (C). With the other hand, grasp the cap (A) and carefully twist the cap of the hard plastic tip counterclockwise (see Figure 1).
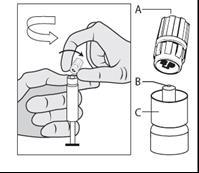
Figure 1
- Remove the cap (A) in a vertical upward direction. To maintain sterility, do not touch the tip of the syringe (B) (see Figure 2).
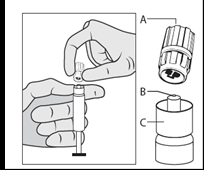
Figure 2
- Attach the needle shield to the Luer-Lok tip and tighten until it is securely locked (see Figure 3).
- Check if the needle is connected to the Luer-Lok tip before tilting it from the vertical position.
- Pull the cap along the needle so as not to damage its sharp end.
- Bring the needle with the cap close to the injection site.
- Remove the cap from the needle.
- Remove any excess air from the syringe.
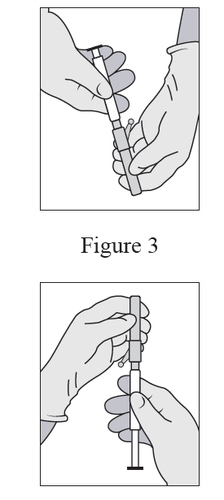
Figure 3
- Administer the medicine intramuscularly, slowly (1-2 minutes/injection) into the buttock muscle. For the convenience of the person administering the injection, the needle bevel is in the same plane as the shield mechanism (see Figure 4).
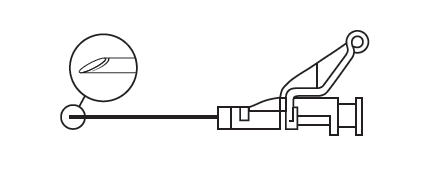
Figure 4
- Immediately after administering the medicine, activate the needle shield by pushing the lever forward with one finger (see Figure 5).
CAUTION: Activate in such a way as to ensure your safety and the safety of others. Listen for a click and visually confirm that the needle tip is completely hidden.
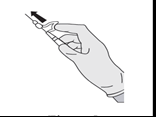
Figure 5
Disposal of residues
Prefilled syringes are intended for single use only.
This medicine may pose a risk to aquatic environments. Any unused product or waste material should be disposed of in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSun Pharmaceutical Industries Europe B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Fulvestrant SunDosage form: Solution, 250 mgActive substance: fulvestrantPrescription not requiredDosage form: Solution, 250 mgActive substance: fulvestrantPrescription requiredDosage form: Solution, 250 mg/ 5 mlActive substance: fulvestrantPrescription required
Alternatives to Fulvestrant Sun in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fulvestrant Sun in Spain
Alternative to Fulvestrant Sun in Ukraine
Online doctors for Fulvestrant Sun
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fulvestrant Sun – subject to medical assessment and local rules.












