

Fulvestrant Sandoz

Ask a doctor about a prescription for Fulvestrant Sandoz

How to use Fulvestrant Sandoz
Package Leaflet: Information for the Patient
Fulvestrant Sandoz, 250 mg/5 ml, Solution for Injection in a Prefilled Syringe
Fulvestrant
Read the Package Leaflet Carefully Before Using the Medication, as it Contains Important Information for the Patient.
- Keep this Package Leaflet, You May Need to Read it Again.
- If You Have Any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
- This Medication Has Been Prescribed for a Specific Person. Do Not Pass it on to Others. The Medication May Harm Another Person, Even if Their Symptoms are the Same.
- If the Patient Experiences Any Side Effects, Including Any Not Listed in this Package Leaflet, Tell Your Doctor, Pharmacist, or Nurse. See Section 4.
Package Leaflet Contents:
- 1. What is Fulvestrant Sandoz and What is it Used for
- 2. Important Information Before Using Fulvestrant Sandoz
- 3. How to Use Fulvestrant Sandoz
- 4. Possible Side Effects
- 5. How to Store Fulvestrant Sandoz
- 6. Package Contents and Other Information
1 What is Fulvestrant Sandoz and What is it Used for
Fulvestrant Sandoz Contains the Active Substance Fulvestrant, Which Belongs to a Group of Medications Called Estrogen Receptor Antagonists.
Estrogens, Female Sex Hormones, May Contribute to the Development of Breast Cancer in Some Cases.
Fulvestrant Sandoz is Used:
- As a Single Medication for the Treatment of Postmenopausal Women with a Certain Type of Breast Cancer, Known as Hormone Receptor-Positive Breast Cancer, Which is Locally Advanced or Has Spread to Other Parts of the Body (Metastatic),
- In Combination with Palbociclib for the Treatment of Women with a Certain Type of Breast Cancer, Known as Hormone Receptor-Positive Breast Cancer, Without Overexpression of Human Epidermal Growth Factor Receptor 2, Which is Locally Advanced or Has Spread to Other Parts of the Body (Metastatic). Pre-Menopausal Women Will Also Receive a Medication Called a Luteinizing Hormone-Releasing Hormone (LHRH) Agonist.
When Fulvestrant Sandoz is Administered with Palbociclib, it is Essential to Also Read the Package Leaflet for the Medication Containing Palbociclib. If You Have Any Questions About Palbociclib, Ask Your Doctor.
2. Important Information Before Using Fulvestrant Sandoz
When Not to Use Fulvestrant Sandoz
If the Patient is Allergic to Fulvestrant or Any of the Other Ingredients of this Medication (Listed in Section 6);
If the Patient is Pregnant or Breastfeeding;
If the Patient Has Severe Liver Impairment.
Warnings and Precautions
Before Using Fulvestrant Sandoz, Discuss with Your Doctor, Pharmacist, or Nurse if Any of the Following Health Problems Apply to You:
Kidney or Liver Impairment;
Low Platelet Count (Involved in the Blood Clotting Process) or Bleeding Disorders;
History of Blood Clots;
Osteoporosis (Decreased Bone Density);
Alcoholism.
Children and Adolescents
Fulvestrant Sandoz is Not Intended for Use in Children and Adolescents Under 18 Years of Age.
Fulvestrant Sandoz and Other Medications
Tell Your Doctor or Pharmacist About All Medications You Are Currently Taking or Have Recently Taken, as Well as Any Medications You Plan to Take.
In Particular, Inform Your Doctor About the Use of Anticoagulant Medications (Medications that Prevent Blood Clots).
Pregnancy and Breastfeeding
If You Are Pregnant, Breastfeeding, Think You May Be Pregnant, or Plan to Have a Child, Consult Your Doctor Before Using this Medication.
Fulvestrant Sandoz Should Not Be Used During Pregnancy. Women of Childbearing Age Should Use Effective Contraception During Treatment and for 2 Years After the Last Dose.
Do Not Breastfeed While Using Fulvestrant Sandoz.
Driving and Operating Machinery
The Effect of Fulvestrant Sandoz on the Ability to Drive and Operate Machinery is Not Expected. However, if Treatment Causes Fatigue, You Should Not Engage in These Activities.
Fulvestrant Sandoz Contains Ethanol, Benzyl Alcohol, and Benzyl Benzoate
This Medication Contains 1000 mg of Ethanol (96% Ethanol) per Dose, Equivalent to 100 mg/ml (10% w/v). The Amount of Ethanol in a Dose of this Medication is Equivalent to Less Than 24 ml of Beer or 10 ml of Wine. The Small Amount of Ethanol in this Medication is Not Expected to Have Noticeable Effects.
This Medication Contains 1000 mg of Benzyl Alcohol per Dose, Equivalent to 100 mg/ml.
Benzyl Alcohol May Cause Allergic Reactions.
Administration of Benzyl Alcohol to Newborns (Up to 4 Weeks of Age) is Associated with a Risk of Severe Side Effects, Including Respiratory Distress (So-Called "Gasping Syndrome").
Do Not Administer to Newborns (Up to 4 Weeks of Age) Without Medical Advice.
Do Not Administer to Young Children (Under 3 Years of Age) for More Than a Week Without Medical Advice or Consultation with a Pharmacist.
Patients with Liver or Kidney Disease Should Consult Their Doctor Before Using the Medication, as High Amounts of Benzyl Alcohol May Accumulate in Their Body and Cause Side Effects (So-Called Metabolic Acidosis).
This Medication Contains 1500 mg of Benzyl Benzoate per Dose, Equivalent to 150 mg/ml.
Benzyl Benzoate May Increase the Risk of Jaundice (Yellowing of the Skin and Eyes) in Newborns (Up to 4 Weeks of Age).
3. How to Use Fulvestrant Sandoz
This Medication Should Always Be Used in Accordance with the Recommendations of Your Doctor or Pharmacist. If You Have Any Doubts, Consult Your Doctor or Pharmacist.
The Recommended Dose of Fulvestrant is 500 mg (Two Injections of 250 mg), Administered Once a Month, and an Additional Dose of 500 mg Administered 2 Weeks After the First Dose.
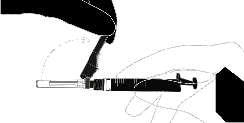
Fulvestrant Sandoz is Administered by a Doctor or Nurse Through a Slow Intramuscular Injection, One in Each Buttock.
If You Have Any Further Questions About the Use of this Medication, Consult Your Doctor, Pharmacist, or Nurse.
4. Possible Side Effects
Like All Medications, Fulvestrant Sandoz Can Cause Side Effects, Although Not Everybody Gets Them.
In Case of Any of the Following Side Effects, Immediate Medical Attention May Be Required:
Allergic Reactions (Hypersensitivity), Including Swelling of the Face, Lips, Tongue, and (or) Throat, Which May Be Symptoms of Anaphylaxis
Blood Clots with Embolism (Increased Risk of Blood Clots)*
Hepatitis
Liver Failure
Tell Your Doctor, Pharmacist, or Nurse If You Experience Any of the Following Side Effects:
Very Common Side Effects(May Occur in More Than 1 in 10 People)
Injection Site Reaction, Such as Pain and (or) Inflammation;
Abnormal Liver Enzyme Activity (Detected in Blood Tests)*
Nausea
Weakness, Fatigue*
Joint and Bone Pain
Hot Flashes
Skin Rash
Allergic Reactions (Hypersensitivity), Including Swelling of the Face, Lips, Tongue, and (or) Throat
All Other Side Effects
Common Side Effects(May Occur in Less Than 1 in 10 People)
Headache
Vomiting, Diarrhea, or Loss of Appetite*
Urinary Tract Infections
Back Pain*
Increased Bilirubin Levels (Liver Pigment Produced in the Liver)
Blood Clots with Embolism (Increased Risk of Blood Clots)*
Decreased Platelet Count (Thrombocytopenia)
Vaginal Bleeding
Lower Back Pain Radiating to One Leg (Sciatica)
Sudden Weakness, Numbness, Tingling, or Loss of Mobility in a Leg (Especially on One Side of the Body), Sudden Difficulty Walking or Maintaining Balance (Peripheral Neuropathy)
Uncommon Side Effects(May Occur in Less Than 1 in 100 People):
Thick, White Vaginal Discharge and Thrush (Infection)
Formation of a Hematoma and Bleeding at the Injection Site
Increased Activity of the Liver Enzyme Gamma-GT (Detected in Blood Tests)
Hepatitis
Liver Failure
Numbness, Tingling, and Pain
Anaphylactic Reactions
* Side Effects in Which the Exact Role of Fulvestrant Sandoz is Not Established Due to the Underlying Disease
Reporting Side Effects
If You Experience Any Side Effects, Including Any Not Listed in this Package Leaflet, Tell Your Doctor, Pharmacist, or Nurse. Side Effects Can Be Reported Directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181 C, 02-222 Warsaw
Phone: +48 22 49 21 301/Fax: +48 22 49 21 309/Website: https://smz.ezdrowie.gov.pl
Side Effects Can Also Be Reported to the Marketing Authorization Holder.
Reporting Side Effects Will Help to Gather More Information on the Safety of the Medication.
5. How to Store Fulvestrant Sandoz
Store the Medication Out of Sight and Reach of Children.
Do Not Use this Medication After the Expiration Date Stated on the Carton or Label of the Prefilled Syringe After: EXP. The Expiration Date Refers to the Last Day of the Specified Month.
This Medication Does Not Require Any Special Storage Conditions.
Medical Personnel Are Responsible for the Proper Storage, Use, and Disposal of the Packaging of the Used Fulvestrant Sandoz Medication.
Do Not Use the Medication if You Notice Any Signs of Damage to the Prefilled Syringe or Spoilage of its Contents, Such as Cloudiness of the Solution, Particles Floating in it, or a Change in Color.
Medications Should Not Be Disposed of in Sewage or Household Waste. Ask Your Pharmacist How to Dispose of Medications That Are No Longer Needed. This Will Help Protect the Environment.
6. Package Contents and Other Information
What Fulvestrant Sandoz Contains
The Active Substance is Fulvestrant.
One Prefilled Syringe Contains 250 mg of Fulvestrant in 5 ml of Solution (50 mg/ml).
The Other Ingredients are: Ethanol (96%), Benzyl Alcohol, Benzyl Benzoate, and Castor Oil.
What Fulvestrant Sandoz Looks Like and What the Package Contains
Fulvestrant Sandoz is a Clear, Colorless to Yellowish, Viscous Solution for Injection in a Prefilled Syringe.
The Package Contains One or Two Single Prefilled Syringes. Additionally, the Package Contains a Sterile Needle.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
Manufacturer
Lek Pharmaceuticals d.d.
Verovškova 57
1526 Ljubljana, Slovenia
EBEWE Pharma Ges.m.b.H. Nfg.KG
Mondseestrasse 11
4866 Unterach, Austria
Fareva Unterach GmbH
Mondseestraße 11
4866 Unterach, Austria
For More Detailed Information on the Medication and Its Names in the Member States of the European Economic Area, Please Contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
Phone: 22 209 70 00
Date of Last Update of the Package Leaflet:01/2022
Sandoz Logo
Information Intended Exclusively for Healthcare Professionals:
The Dose of Fulvestrant Sandoz 500 mg (2 x 250 mg/5 ml Solution for Injection) Should Be Administered Using Two Prefilled Syringes (See Section 3).
Administration Instructions
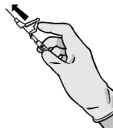
Warning – Do Not Sterilize the Needle with an Autoclave Before Use.
Avoid Hand Contact with the Needle During Use and Disposal.
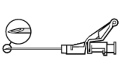
The Syringes are Supplied with a Needle with a Safety System BD SafetyGlide or Terumo SurGuard .
Instructions for the BD SafetyGlide Needle:
| |
| |
| |
|  |
| |
|
| |
|  |
|  |
| WARNING: Proceed in a Way That Ensures Your Safety and the Safety of Others. Listen for the Click and Visually Verify That the Needle Tip is Completely Covered. | |
Instructions for the Terumo SurGuard Needle:
Applicable to Each Syringe:
| |
|  |
|  |
| |
| |
| |
| 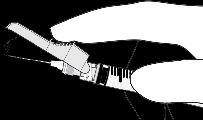 |
| o Activation with the Index Finger | |
| o Activation with the Thumb | 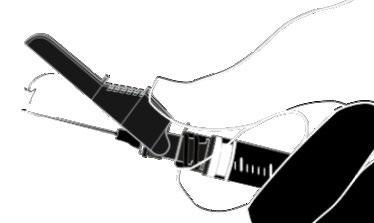 |
| o Activation Against the Surface | 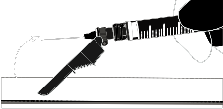 |
| Activation is Confirmed by an Audible and (or) Tactile Click, and Can Also Be Visually Verified. In Case of Doubt Whether the Shield Completely Covers the Needle, Repeat the Final Step. | |
Disposal
The Prefilled Syringes are Intended Exclusivelyfor Single Use.
Any Unused Medication or Waste Should Be Disposed of in Accordance with Local Regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterEbewe Pharma Ges.m.b.H Nfg. KG Fareva Unterach GmbH LEK Pharmaceuticals d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Fulvestrant SandozDosage form: Solution, 250 mgActive substance: fulvestrantPrescription not requiredDosage form: Solution, 250 mgActive substance: fulvestrantPrescription requiredDosage form: Solution, 250 mg/ 5 mlActive substance: fulvestrantPrescription required
Alternatives to Fulvestrant Sandoz in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fulvestrant Sandoz in Spain
Alternative to Fulvestrant Sandoz in Ukraine
Online doctors for Fulvestrant Sandoz
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fulvestrant Sandoz – subject to medical assessment and local rules.












