
Fulvestrant Fresenius Kabi
Ask a doctor about a prescription for Fulvestrant Fresenius Kabi

How to use Fulvestrant Fresenius Kabi
Package Leaflet: Information for the User
Fulvestrant Fresenius Kabi, 250 mg/5 ml, Solution for Injection in a Prefilled Syringe
Fulvestrant
Read All of This Leaflet Carefully Before Using This Medicine Because It Contains Important Information for You.
- Keep This Leaflet. You May Need to Read It Again.
- If You Have Any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
- This Medicine Has Been Prescribed for You Only. Do Not Pass It on to Others. It May Harm Them, Even if Their Signs of Illness Are the Same as Yours.
- If You Experience Any Side Effects, Tell Your Doctor, Pharmacist, or Nurse. See Section 4.
Contents of the Pack
- 1. What Fulvestrant Fresenius Kabi Is and What It Is Used For
- 2. Important Information Before You Use Fulvestrant Fresenius Kabi
- 3. How to Use Fulvestrant Fresenius Kabi
- 4. Possible Side Effects
- 5. How to Store Fulvestrant Fresenius Kabi
- 6. Contents of the Pack and Other Information
1. What Fulvestrant Fresenius Kabi Is and What It Is Used For
Fulvestrant Fresenius Kabi Contains the Active Substance Fulvestrant, Which Belongs to a Group of Medicines Called Estrogen Receptor Antagonists. Estrogens, Female Sex Hormones, Can Sometimes Influence the Growth of Breast Cancer. Fulvestrant Fresenius Kabi Is Used:
- As a Single Medicine, in the Treatment of Postmenopausal Women with a Certain Type of Breast Cancer Called Hormone Receptor-Positive Breast Cancer, Which Is Locally Advanced or Has Spread to Other Parts of the Body (Metastatic) or
- In Combination with Palbociclib in the Treatment of Women with a Certain Type of Breast Cancer Called Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2 (HER2)-Negative Breast Cancer, Which Is Locally Advanced or Has Spread to Other Parts of the Body (Metastatic). Women Who Have Not Yet Reached Menopause Will Also Receive a Medicine Called a Luteinizing Hormone-Releasing Hormone (LHRH) Agonist.
When Fulvestrant Fresenius Kabi Is Given in Combination with Palbociclib, It Is Also Important to Read the Package Leaflet for Palbociclib. If You Have Any Questions About Palbociclib, Ask Your Doctor.
2. Important Information Before You Use Fulvestrant Fresenius Kabi
When Not to Use Fulvestrant Fresenius Kabi
- If You Are Allergic to Fulvestrant or Any of the Other Ingredients of This Medicine (Listed in Section 6);
- If You Are Pregnant or Breast-Feeding;
- If You Have Severe Liver Problems.
Warnings and Precautions
Tell Your Doctor, Pharmacist, or Nurse Before Using Fulvestrant Fresenius Kabi If You Have Ever Had Any of the Following Health Problems:
- Kidney or Liver Disease;
- Low Platelet Count (Which Helps the Blood to Clot) or Bleeding Disorders;
- Blood Clots;
- Osteoporosis (Problems with the Mineral Density of Your Bones);
- Alcohol Dependence.
Children and Adolescents
Fulvestrant Fresenius Kabi Is Not Recommended for Use in Children and Adolescents Under 18 Years of Age.
Fulvestrant Fresenius Kabi and Other Medicines
Tell Your Doctor or Pharmacist About All Medicines You Are Taking or Have Recently Taken, as Well as Any Medicines You Plan to Take. In Particular, Tell Your Doctor If You Are Taking Blood-Thinning Medicines (Medicines That Prevent the Formation of Blood Clots).
Pregnancy and Breast-Feeding
Fulvestrant Fresenius Kabi Must Not Be Used During Pregnancy. If You Are Able to Become Pregnant, You Should Use Effective Contraception During Treatment with Fulvestrant Fresenius Kabi and for 2 Years After the Last Dose. You Must Not Breast-Feed While Using Fulvestrant Fresenius Kabi.
Driving and Using Machines
Fulvestrant Has Not Been Shown to Affect the Ability to Drive or Use Machines. However, If You Feel Tired After Receiving This Medicine, Do Not Drive or Use Machines.
Fulvestrant Fresenius Kabi Contains 500 Mg of Ethanol (Alcohol) per Injection
This Is Equivalent to 100 Mg/Ml (10% V/V). The Amount of Alcohol in Each Dose of This Medicine Is Equivalent to 13 Ml of Beer or 5 Ml of Wine. The Amount of Alcohol in This Medicine Is Unlikely to Have Any Effect on Adults and Adolescents. However, the Alcohol in This Medicine May Affect the Way Other Medicines Work. If You Are Taking Other Medicines, You Should Consult Your Doctor or Pharmacist. If You Are Pregnant or Breast-Feeding, You Should Consult Your Doctor or Pharmacist Before Using This Medicine. If You Are Alcohol Dependent, You Should Consult Your Doctor or Pharmacist Before Using This Medicine.
Fulvestrant Fresenius Kabi Contains 500 Mg of Benzyl Alcohol per Injection
This Is Equivalent to 100 Mg/Ml. Benzyl Alcohol May Cause Allergic Reactions.
Fulvestrant Fresenius Kabi Contains 750 Mg of Benzyl Benzoate per Injection
This Is Equivalent to 150 Mg/Ml.
3. How to Use Fulvestrant Fresenius Kabi
Fulvestrant Fresenius Kabi Is Given by a Doctor or Nurse. The Medicine Will Be Injected Slowly into a Muscle in Two Separate Injections of 5 Ml, Each into a Different Buttock. The Recommended Dose Is 500 Mg of Fulvestrant (Two Injections of 250 Mg/5 Ml), Given Once a Month, and an Additional Dose of 500 Mg Given 2 Weeks After the First Dose. If You Have Any Further Questions on the Use of This Medicine, Ask Your Doctor, Pharmacist, or Nurse.
4. Possible Side Effects
Like All Medicines, Fulvestrant Fresenius Kabi Can Cause Side Effects, Although Not Everybody Gets Them.
Severe Side Effects
If You Experience Any of the Following Side Effects, You Must Contact a Doctor Immediately:
- Allergic Reactions (Hypersensitivity), Including Swelling of the Face, Lips, Tongue, and/or Throat, Which May Be Symptoms of Anaphylactic Reactions;
- Blood Clots (Increased Risk of Venous Thromboembolism);
- Liver Inflammation;
- Liver Failure.
If You Experience Any of the Following Side Effects, You Should Tell Your Doctor, Pharmacist, or Nurse:
Very Common Side Effects (May Affect More Than 1 in 10 People):
- Pain and/or Inflammation at the Injection Site;
- Changes in Liver Enzyme Activity (in Blood Tests);
- Nausea;
- Feeling Weak, Tired;
- Joint and Muscle Pain;
- Hot Flushes;
- Rash;
- Allergic Reactions (Hypersensitivity), Including Swelling of the Face, Lips, Tongue, and/or Throat.
All Other Side Effects:
Common Side Effects (May Affect Up to 1 in 10 People):
- Headache;
- Vomiting, Diarrhea, or Loss of Appetite;
- Urinary Tract Infections;
- Back Pain;
- Increased Bilirubin Levels (a Pigment Produced by the Liver);
- Blood Clots (Increased Risk of Venous Thromboembolism);
- Low Platelet Count (Thrombocytopenia);
- Vaginal Bleeding;
- Lower Back Pain Radiating to One Leg (Sciatica);
- Sudden Weakness, Numbness, Tingling, or Loss of Mobility in a Leg, Especially on One Side of the Body, Sudden Difficulty Walking or Maintaining Balance (Peripheral Neuropathy).
Uncommon Side Effects (May Affect Up to 1 in 100 People):
- Thick, White Vaginal Discharge and Vaginal Candidiasis (Infection);
- Bluish Discoloration and Bleeding at the Injection Site;
- Increased Activity of the Liver Enzyme Gamma-Glutamyltransferase in Blood Tests;
- Liver Inflammation (Hepatitis);
- Liver Failure;
- Numbness, Tingling, and Pain;
- Anaphylactic Reactions.
Reporting of Side Effects
If You Experience Any Side Effects, Tell Your Doctor, Pharmacist, or Nurse. Side Effects Can Also Be Reported Directly to the Pharmacovigilance Department of the Medicines Agency. By Reporting Side Effects, You Can Help Provide More Information on the Safety of This Medicine.
5. How to Store Fulvestrant Fresenius Kabi
Keep This Medicine Out of the Sight and Reach of Children. Do Not Use This Medicine After the Expiry Date Which Is Stated on the Carton After EXP. The Expiry Date Refers to the Last Day of That Month. Store and Transport at a Refrigerated Temperature (2°C - 8°C). Limit Storage at Temperatures Other Than 2°C - 8°C. Avoid Storage at Temperatures Above 30°C and Do Not Exceed 28 Days with an Average Storage Temperature Below 25°C (but Above the Range 2°C - 8°C). If the Temperature Range Is Exceeded, Apply the Recommended Storage Conditions Immediately (Store and Transport in a Refrigerator at 2°C - 8°C). Exceeding the Appropriate Storage Temperature May Have a Cumulative Effect on the Quality of the Medicine, and the 28-Day Period Must Not Be Exceeded Within the Shelf Life of Fulvestrant Fresenius Kabi (See Section 6.3). Exposure to a Temperature Below 2°C Does Not Damage the Medicine, Provided It Is Not Stored Below -20°C. Store the Prefilled Syringe in the Original Package to Protect from Light. Healthcare Professionals Are Responsible for the Proper Storage, Handling, and Disposal of Fulvestrant Fresenius Kabi. This Medicine May Pose a Risk to the Aquatic Environment. Medicines Should Not Be Disposed of via Wastewater or Household Waste. Ask Your Pharmacist How to Dispose of Medicines No Longer Required. These Measures Will Help Protect the Environment.
6. Contents of the Pack and Other Information
What Fulvestrant Fresenius Kabi Contains
- The Active Substance Is Fulvestrant. Each Prefilled Syringe (5 Ml) Contains 250 Mg of Fulvestrant.
- The Other Ingredients Are Ethanol (96%), Benzyl Alcohol, Benzyl Benzoate, and Purified Castor Oil.
What Fulvestrant Fresenius Kabi Looks Like and Contents of the Pack
Fulvestrant Fresenius Kabi Is a Clear, Colorless to Yellowish, Viscous Solution for Injection in a Prefilled Syringe Made of Colorless Glass Type I, with a Piston Made of Polystyrene and a Needle Shielding System, in a Cardboard Box Containing 5 Ml of Fulvestrant Solution. Two Prefilled Syringes Are Required to Administer the Recommended Monthly Dose of 500 Mg. Fulvestrant Fresenius Kabi Is Available in Three Pack Sizes Containing 1, 2, or 6 Prefilled Syringes. The Packs Also Contain 1, 2, or 6 Needles with a Safety System (BD SafetyGlide). Not All Pack Sizes May Be Marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
Manufacturer
Laboratorios Farmalán, S.A.
Calle La Vallina s/n, Edificio 2
Polígono Industrial Navatejera
24193, Villaquilambre, León
Spain
This Medicine Is Authorized in the Member States of the European Economic Area Under the Following Names:
| Austria | Fulvestrant Fresenius Kabi 250 mg Injektionslösung in einer Fertigspritze |
| Belgium | Fulvestrant Fresenius Kabi 250 mg oplossing voor injectie in een voorgevulde spuit, solution injectable en seringue préremplie, Injektionslösung in einer Fertigspritze |
| Czech Republic | Fulvestrant Fresenius Kabi |
| Denmark | Fulvestrant Fresenius Kabi |
| Finland | Fulvestrant Fresenius Kabi 250 mg injektioneste, liuos, esitäytetty ruisku |
| Germany | Fulvestrant Fresenius Kabi 250 mg Injektionslösung in einer Fertigspritze |
| Italy | Fulvestrant Fresenius Kabi |
| Netherlands | Fulvestrant Fresenius Kabi 250 mg, oplossing voor injectie in een voorgevulde spuit |
| Norway | Fulvestrant Fresenius Kabi |
| Poland | Fulvestrant Fresenius Kabi |
| Portugal | Fulvestrant Fresenius Kabi |
| Slovenia | Fulvestrant Fresenius Kabi 250 mg raztopina za injiciranje v napolnjeni injekcijski brizgi |
| Sweden | Fulvestrant Fresenius Kabi 250 mg injektionsvätska, lösning i förfylld spruta |
| Slovakia | Fulvestrant Fresenius Kabi 250 mg |
| United Kingdom | Fulvestrant Fresenius Kabi 250 mg solution for injection in pre-filled syringe |
Date of Last Revision of the Leaflet: --------------------------------------------------------------------------------------------------------------------
Information Intended for Healthcare Professionals Only:
Fulvestrant Fresenius Kabi 500 Mg (2 x 250 Mg/5 Ml Solution for Injection) Should Be Administered Using Two Prefilled Syringes, See Section 3. Administration Instructions Note - Do Not Place the Needle with the Safety System (BD SafetyGlide Shielding Hypodermic Needle) in an Autoclave Before Use. When Handling the Medicine and Disposing of Waste, Avoid Hand Contact with the Needle. Applies to Both Syringes:
- Remove the Glass Syringe from the Container and Check for Damage.
- Open the Outer Package of the Needle with the Safety System (SafetyGlide).
- Before Administering Parenteral Solutions, Visually Inspect for Particulate Matter and Discoloration.
- Hold the Syringe Upright in the Fluted Part (C). With the Other Hand, Grasp the Needle Shield (A) and Carefully Twist It Forward and Then Twist the Plastic Needle Shield in a Counter-Clockwise Direction (See Figure 1).
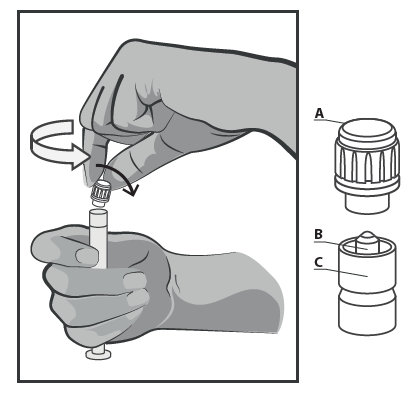
Figure 1
- Remove the Needle Shield (A) in the Upright Position. To Maintain Sterility, Do Not Touch the Needle Tip (B) (See Figure 2).
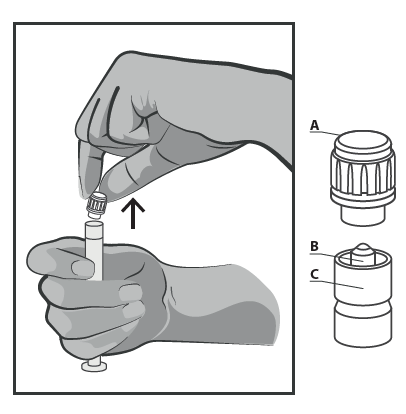
Figure 2
- Attach the Needle with the Safety System to the Luer-Lock Tip and Tighten to Secure (See Figure 3).
- Check That the Needle Is Properly Attached to the Luer-Lock Tip Before Proceeding to the Upright Position.
- When Tightening the Needle, Be Careful Not to Damage the Sharp Needle Tip.
- Bring the Needle with the Safety System Close to the Injection Site.
- Remove the Needle Shield.
- Remove Any Air from the Syringe.
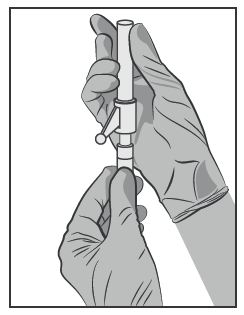
Figure 3
- Administer the Medicine Intramuscularly, Slowly (1-2 Minutes/Injection), into the Buttock Muscle (Buttock Site). For the Convenience of the Person Administering the Medicine, the Needle Bevel Is on the Same Surface as the Safety Mechanism (See Figure 4).
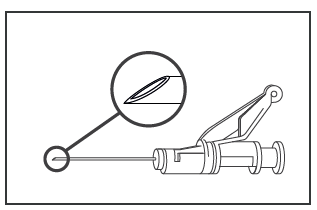
Figure 4
- Immediately After Injection, Activate the Needle Safety Mechanism by Pushing the Activator Forward (See Figure 5).
WARNING: Proceed with Caution to Ensure Your Safety and the Safety of Others. Listen for the Click and Visually Confirm That the Needle Tip Is Fully Enclosed.
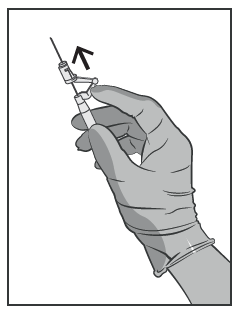
Figure 5
Disposal of Waste the Prefilled Syringes Are for Single Use Only. This Medicine May Pose a Risk to the Aquatic Environment. Any Unused Medicinal Product or Waste Material Should Be Disposed of in Accordance with Local Requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratorios Farmalán, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Fulvestrant Fresenius KabiDosage form: Solution, 250 mgActive substance: fulvestrantPrescription not requiredDosage form: Solution, 250 mgActive substance: fulvestrantPrescription requiredDosage form: Solution, 250 mg/ 5 mlActive substance: fulvestrantPrescription required
Alternatives to Fulvestrant Fresenius Kabi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fulvestrant Fresenius Kabi in Spain
Alternative to Fulvestrant Fresenius Kabi in Ukraine
Online doctors for Fulvestrant Fresenius Kabi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fulvestrant Fresenius Kabi – subject to medical assessment and local rules.












