
How to use Finiuve
Package Leaflet: Information for the Patient
Finjuve, 2.275 mg/ml, spray for the skin, solution
Finasteride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Finjuve and what is it used for
- 2. Important information before using Finjuve
- 3. How to use Finjuve
- 4. Possible side effects
- 5. How to store Finjuve
- 6. Contents of the pack and other information
1. What is Finjuve and what is it used for
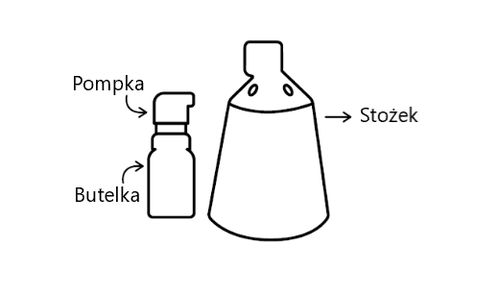
Finjuve contains finasteride as the active substance. It is applied to the scalp, where hair loss occurs, using a spray applicator consisting of a bottle with a pump and a cone.
Finjuve is used to treat mild to moderate male pattern hair loss (also known as androgenetic alopecia). Finjuve increases hair growth on the scalp and prevents further hair loss in men. Finjuve is intended for use in men aged 18 to 41 years.
Male pattern hair loss is a common problem. It is thought that the combination of genetic factors and a specific hormone, dihydrotestosterone (DHT), plays a significant role in its causes. In male pattern hair loss, the scalp has increased amounts of DHT. This hormone is thought to contribute to shortening the time hair grows and thinning hair.
The hair follicles become smaller (miniaturized), and hair loss becomes more visible.
Finjuve reduces the concentration of DHT in the scalp. This helps to reverse the hair loss process, leading to increased hair growth and preventing further hair loss.
2. Important information before using Finjuve
When not to use Finjuve
- in women who are pregnant or may become pregnant. Finjuve must not be used in women.
Warnings and precautions
Before starting to use Finjuve, discuss it with your doctor or pharmacist.
Transfer of Finjuve
This medicine may cause birth defects of the genital organs in a male child if the active substance of the medicine, finasteride, is absorbed through the skin into a pregnant woman's body. Pregnant or potentially pregnant women should avoid skin contact with the treated area.
Women should also be told not to touch surfaces that may have come into contact with Finjuve. If contact with Finjuve occurs, the woman should immediately wash the affected area of the body with soap and water.
Children and adolescents must not come into contact with Finjuve. However, if contact with the medicine occurs, they should immediately wash the affected area of the body with soap and water.
Effect on prostate-specific antigen (PSA)
If a patient is undergoing a prostate-specific antigen (PSA) blood test as a screening test for prostate cancer, the patient should tell the doctor that they are using Finjuve, as this may be relevant for the interpretation of the test results.
Effect on male hormone dihydrotestosterone (DHT)
Finjuve reduces the concentration of the male hormone DHT in the blood, often to below normal values. However, this occurs less frequently, and the decrease in concentration is smaller than with oral finasteride. Adverse reactions of a sexual nature, known to occur with oral finasteride, may also occur with Finjuve, but this is less likely (see section 4). Therefore, the doctor's dosage recommendations should be followed. Do not use more than 4 sprays per day.
Breast cancer
Although breast cancer has not been observed in men treated with Finjuve, such cases have been reported during treatment with oral finasteride. If any changes in the breasts are noticed, such as lumps, pain, enlargement of the breasts, or discharge from the nipples, the doctor should be consulted as soon as possible.
Mood changes and depression
Although mood changes have not been observed in patients treated with Finjuve, they have been reported during treatment with oral finasteride. If symptoms such as low mood, depression, or suicidal thoughts occur, the doctor should be consulted as soon as possible.
Children and adolescents
Finjuve must not be used in children and adolescents. There are no data on the efficacy and safety of finasteride in children and adolescents under 18 years of age.
Finjuve and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
If you are using other topical products, such as cosmetics, products containing sunscreens, or other topical medicines, you should not use Finjuve on the same area of skin.
Pregnancy
Finjuve must not be used in women.
Pregnant or potentially pregnant women must avoid skin contact with the area treated with Finjuve or other surfaces that may have come into contact with Finjuve. See the section "Transfer of Finjuve" above. If direct contact with the medicine occurs, the woman should immediately wash the affected area of the body with soap and water and consult a doctor.
Driving and using machines
Finjuve has no effect on the ability to drive or use machines.
Finjuve contains ethanol
This medicine contains 25 mg of ethanol (96%) per spray, which corresponds to a concentration of 0.5 mg/microliter (55%). This may cause a burning sensation on damaged skin.
3. How to use Finjuve
This medicine should always be used exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Depending on the size of the area of the scalp affected by hair loss, your doctor will recommend 1 to 4 sprays per day. Do not use more than 4 sprays per day.
Finjuve is for use on the skin only. It should only be applied to the scalp.
Finjuve consists of 2 separate parts: a bottle with a pump and a cone. These parts need to be assembled before the first use of the medicine. Before using the medicine for the first time, read the entire instruction manual for the device presented below.
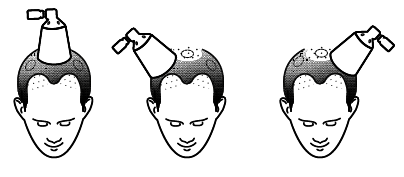
Before using the medicine, make sure your hair and scalp are completely dry. Finjuve should be applied by the patient themselves. If more than 1 spray is recommended, subsequent sprays should be applied to non-overlapping areas of the scalp.
Do not spray the solution onto other areas of the body besides the scalp. After applying Finjuve, leave it on the scalp for at least 6 hours.
It is possible to transfer Finjuve through contact with clothing, hands, or other surfaces and objects. Avoid contact between the treated scalp and a pillow, helmet, cap, etc. until the scalp is dry.
Finjuve can be transferred from the patient's body to another person if they touch the treated scalp or other surfaces that have come into contact with the medicine. If such contact occurs, the person should immediately wash the affected area of the body with soap and water.
Finjuve should be stored in a safe place out of the reach of children. Family members and other individuals with access to the place where the medicine is stored should be informed about the need to take precautions when coming into contact with the medicine.
Components and assembly of the spray applicator
Assembly of the spray applicator
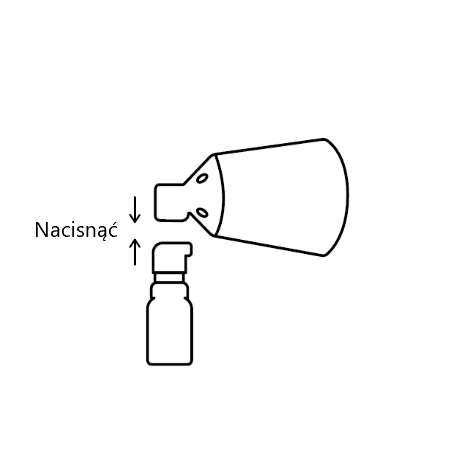
AAlign and press
Align the cone to the pump button and press firmly.
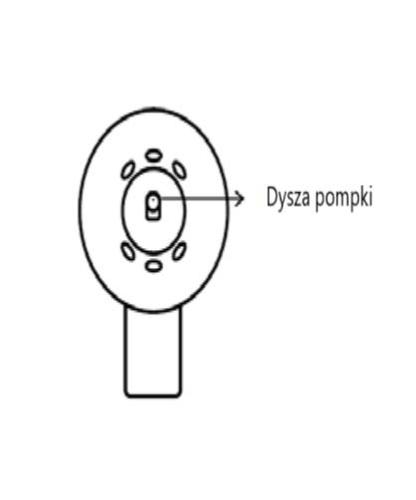
BCorrect assembly
The spray applicator is correctly assembled if, when pressed, you hear a click and the spray button is in the central position of the cone,
CCorrect assembly
the lower part of the pump button is aligned with the lower part of the cone, without any gap between them.
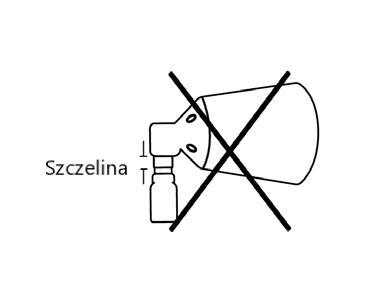
DIncorrect assembly, gap. Align the parts and press again.
If, during assembly, you do not hear a click or see a gap between the lower part of the pump button and the cone, align the parts and press again.
Activating the pump
- After assembling the spray applicator, before its first use, check the pump's operation. If the spray applicator has not been used for 2 weeks or longer, the pump's operation will need to be checked again. There is no need to check the pump's operation before each use.
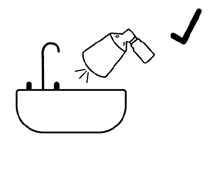
- To check the pump's operation for the first time, press the pump 4 times with your thumb or index finger, directing the spray to the sink. Then rinse the sink with water. To check the pump's operation again after a period of non-use of 2 weeks or longer, press the pump only 1 time.
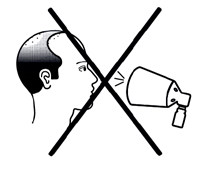
- Do not spray Finjuve towards your face.
- If, during assembly or operation of the device, the solution is released, wash the surface where it may have accumulated.
Checking the pump's operation
Do not spray the medicine towards your face
Dose application
- Depending on the size of the area of the scalp affected by hair loss, your doctor will recommend 1 to 4 sprays per day.
- There is no need to shake the bottle before using the medicine.
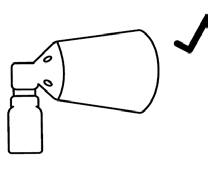
- Hold the spray applicator so that the cone is in contact with the scalp, which will help prevent the solution from being dispersed in the air,
- Press the pump 1 time to the end to obtain 1 spray.
- Move the cone to other areas of the scalp to apply subsequent doses, depending on the number of sprays recommended by your doctor. The area of subsequent application should not overlap with the area where the medicine has already been applied.
- After applying the medicine, do not remove the cone from the pump. Put the spray applicator back in the box.
- After application, do not wash Finjuve off for at least 6 hours.
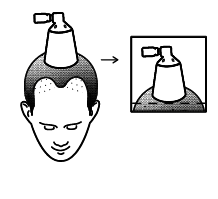
The cone is in contact with the scalp
Move the cone over the next area of the scalp
so that the treated areas do not overlap with each other
Try to avoid contact between your hands or other parts of your body and Finjuve. Any other parts of the body that have come into contact with Finjuve should be washed immediately with soap and water.
If the cone becomes soiled, wipe it with a clean, dry cloth. Dispose of the cloth safely and wash your hands thoroughly.
Dose and treatment days depending on the dose
The bottle contains up to 180 sprays. The number of treatment days depends on the prescribed dose, which may be 1 to 4 sprays per day. Do not use the medicine from the bottle after 180 sprays have been used, as the remaining amount of solution in the bottle may not provide a full dose, which may reduce the treatment effect.
| Number of sprays per day | Treatment days |
| 1 | 180 |
| 2 | 90 |
| 3 | 60 |
| 4 | 45 |
- The recommended dose and associated treatment days for complete use of the medicine will be indicated on the carton by the pharmacist.
- On the day you start treatment with Finjuve, make a note in your calendar of the recommended dose (1 to 4 sprays) and calculate when a new bottle of medicine will be needed. Before the medicine runs out, contact your doctor to avoid interrupting treatment.
Using more than the recommended dose of Finjuve
If you use more Finjuve than recommended, consult your doctor. Finjuve will not work faster or better if used more often than once a day, but the risk of side effects may increase.
Missing a dose of Finjuve
If you miss a dose of Finjuve, do not use a double dose to make up for the missed applications. Continue to use the medicine at the dose recommended by your doctor.
Stopping treatment with Finjuve
Treatment effects may appear only after 3 months. It is important to continue using Finjuve for as long as your doctor recommends. If treatment with Finjuve is stopped, the regained hair will likely be lost.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common (may affect up to 1 in 10 people)
- Itching or redness of the scalp
Very common (may affect more than 1 in 10 people)
- Decreased concentration of male hormones (dihydrotestosterone) in the blood
During treatment with Finjuve, other side effects observed with oral finasteride may also occur. These include:
- Allergic reactions (hypersensitivity), such as: skin rash, itching, swelling around the mouth (angioedema)
- Low mood
- Anxiety
- Palpitations (heart pounding)
- Increased activity of liver enzymes
- Tenderness and enlargement of the breasts
- Testicular pain
- Blood in the semen (hematospermia)
- Decreased sex drive
- Difficulty getting an erection
- Ejaculation disorders, including reduced semen volume
- Infertility
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Finjuve
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and bottle label after EXP. The expiry date refers to the last day of that month.
There are no special storage instructions for this medicine.
Finjuve contains alcohol and is therefore flammable. Do not spray near an open flame or while smoking.
Shelf life after first opening: 6 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Finjuve contains
- The active substance is finasteride. 1 ml of solution contains 2.275 mg of finasteride. Each spray delivers 50 microliters of solution, which corresponds to 114 micrograms of finasteride.
- The other ingredients are: ethanol 96%, purified water, propylene glycol, hydroxypropyl chitosan.
What Finjuve looks like and contents of the pack
Finjuve is a colorless, clear, slightly viscous solution for cutaneous spray.
Pack sizes:
1 bottle (180 doses) with a mechanical spray pump and separate cone
3 bottles (3 x 180 doses) with a mechanical spray pump and 3 separate cones
Before the first use, attach the cone to the pump of the bottle as described in section 3.
Not all pack sizes may be marketed.
Marketing authorization holder
Polichem S.A.
50, Val Fleuri
L-1526 Luxembourg
Manufacturer
Almirall Hermal GmbH
Scholtzstrasse 1 and 3,
21465, Reinbek,
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany
Finjuve für Männer 2,275 mg/ml Spray zur Anwendung auf der Haut (Kopfhaut), Lösung
Bulgaria
Финюве за мъже 2,275 mg/ml спрей за кожа, разтвор
Finjuve for men 2.275 mg/ml cutaneous spray, solution
Czech Republic
Fynzur 2.275 mg/ml kožní sprej, roztok
Hungary
Fynzur férfiaknak 2,275 mg/ml külsőleges oldatos spray
Italy
CARETOPIC 2.275 mg/ml spray cutaneo, soluzione
Luxembourg
Finjuve für Männer 2,275 mg/ml Spray zur Anwendung auf der Haut (Kopfhaut), Lösung
Poland
Finjuve
Portugal
Finasterida Cantabria 2.275 mg/ml Solução para Pulverização Cutânea
Romania
Finjuve pentru bărbați 2,275 mg/ml spray cutanat, soluție
Slovakia
Finjuve pre mužov 2,275 mg/ml dermálny roztokový sprej
Spain
Alocare 2,275 mg/ml Solución para pulverización cutánea
To obtain more detailed information on this medicine, please contact the local representative of the marketing authorization holder:
Egis Pharmaceuticals PLC
Phone number: +48 22 417 92 00
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAlmirall Hermal GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FiniuveDosage form: Solution, 5%Active substance: finasterideManufacturer: Pierre Fabre Medicament Production Site PROGIPHARMPrescription not requiredDosage form: Tablets, 1 mgActive substance: finasteridePrescription requiredDosage form: Tablets, 1 mgActive substance: finasteridePrescription required
Alternatives to Finiuve in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Finiuve in Spain
Alternative to Finiuve in Ukraine
Online doctors for Finiuve
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Finiuve – subject to medical assessment and local rules.






