
How to use Alopexi
Leaflet accompanying the packaging: patient information
Alopexy, 50 mg/ml, solution for the skin
Minoxidil
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a doctor or pharmacist.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
- If hair loss persists for more than 6 weeks or worsens, consult a doctor.
Table of contents of the leaflet
- 1. What Alopexy, 50 mg/ml, solution for the skin is and what it is used for
- 2. Important information before using Alopexy, 50 mg/ml, solution for the skin
- 3. How to use Alopexy, 50 mg/ml, solution for the skin
- 4. Possible side effects
- 5. How to store Alopexy, 50 mg/ml, solution for the skin
- 6. Contents of the packaging and other information
1. What Alopexy, 50 mg/ml, solution for the skin is and what it is used for
Alopexy is a solution for application to the skin, which contains minoxidil, an active substance that stimulates hair growth.
Alopexy is recommended in certain cases of androgenetic alopecia (excessive hair loss) of moderate severity in men.
It is not recommended to use this medicine in womendue to the frequent occurrence of excessive hair growth outside the application site.
2. Important information before using Alopexy, 50 mg/ml, solution for the skin
When not to use Alopexy, 50 mg/ml, solution for the skin:
- If the patient is allergic to minoxidil or any of the other ingredients of this medicine (listed in section 6).
- If the patient has poorly tolerated 20 mg/ml minoxidil solution.
- If the patient has damage to the scalp.
Children and adolescents
The safety and efficacy of Alopexy, 50 mg/ml, solution for the skin in children and adolescents under 18 years of age have not been established. There are no available data. Therefore, Alopexy, 50 mg/ml, solution for the skin should not be used in children and adolescents under 18 years of age.
Elderly
This medicine is not recommended for use in people over 65 years of age, as its efficacy and safety have not been studied.
Warnings and precautions
Before starting treatment with Alopexy, discuss it with your doctor or pharmacist.
- Particular caution should be exercised if the patient currently has or has had decreased blood pressure (hypotension) or cardiovascular disease or heart rhythm disorders, such as tachycardia, chest pain, loss of consciousness, dizziness, unexplained weight gain or signs of water and/or sodium retention (swelling of hands or feet). In these cases, consult a doctor before using this medicine. Patients should be monitored at the start of treatment and regularly during treatment.
- Due to the risk of excessive hair growth outside the application site, the medicine should not be used in women.
- There have been cases of excessive hair growth on the body of infants following skin contact with minoxidil application sites in patients (caregivers) using topical minoxidil. Hair growth returned to normal within a few months when the infant was no longer exposed to minoxidil. Care should be taken to avoid children coming into contact with areas of the body where minoxidil has been applied topically. If excessive hair growth on the body of a child is observed during the use of topical minoxidil products, consult a doctor.
Do not use this medicine:
- If the patient's relatives do not have hair loss, hair loss is sudden and/or partial, hair loss occurred after childbirth or the cause of hair loss is unknown. In these cases, consult a doctor before using Alopexy, 50 mg/ml, solution for the skin, as treatment may not be effective.
- If the scalp is red, inflamed, infected, irritated, or painful. This medicine should only be used on healthy scalp skin (see "How to use Alopexy, 50 mg/ml, solution for the skin"). If the scalp skin is damaged, increased amounts of the active substance - minoxidil - may penetrate the bloodstream (see "When not to use Alopexy, 50 mg/ml, solution for the skin").
- Do not use this medicine in combination with other skin medicines for the scalp.
- Do not use on other parts of the body.
During treatment
- The medicine contains ethanol (alcohol) and may cause a burning sensation and irritation if it accidentally gets into the eyes, wounds, irritated skin, or mucous membranes: rinse the affected areas thoroughly with cold running water. If irritation persists, consult a doctor.
- Transient worsening of hair loss may occur during the first 2 to 6 weeks of treatment. If hair loss persists for more than 6 weeks or worsens, discontinue use of Alopexy, 50 mg/ml, solution for the skin and consult a doctor.
- Avoid exposing the treated scalp to the sun: wear a hat.
- Do not swallow. If the medicine is swallowed, IMMEDIATELY CONSULT A DOCTOR. Accidental ingestion may cause symptoms resulting from the effect of minoxidil on the cardiovascular system. Therefore, the medicine should be kept out of sight and reach of children.
- Do not inhale the sprayed medicine.
- In some patients, changes in hair color and/or texture have been reported
Stop treatment and consult a doctor immediately:
- If you experience low blood pressure, chest pain, rapid heartbeat.
- In case of fainting or dizziness.
- If you experience sudden, unexplained weight gain, swelling of hands or feet (edema).
- In case of persistent redness or irritation of the scalp.
Alopexy, 50 mg/ml, solution for the skin and other medicines
Do not use this medicine at the same time as other dermatological medicines containing tretinoin, anthralin, or betamethasone dipropionate, as these medicines may alter the amount of minoxidil in the blood.
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take - including those available without a prescription.
Alopexy, 50 mg/ml, solution for the skin with food and drink
Not applicable.
Pregnancy and breastfeeding
It is not recommended to use this medicine in women.
Avoid using this medicine during pregnancy and breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, consult a doctor or pharmacist before using this medicine.
Driving and using machines
Alopexy, 50 mg/ml, solution for the skin has no or negligible influence on the ability to drive and use machines.
Alopexy, 50 mg/ml, solution for the skin contains propylene glycol and ethanol
Each dose of Alopexy contains 240 mg of propylene glycol, which corresponds to 240 mg of propylene glycol per 1 ml of solution.
This medicine contains 520 mg of alcohol (ethanol) per ml, which corresponds to 520 mg of ethanol per 1 ml of solution. The medicine may cause burning of damaged skin.
This medicine contains ethanol and is therefore flammable.
3. How to use Alopexy, 50 mg/ml, solution for the skin
WARNING:
Flammable product.
Keep away from heat sources, hot surfaces, sparks, open flames, and other ignition sources. Do not smoke during application or while holding this medicine.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist. If in doubt, consult a doctor or pharmacist.
Dose
The recommended dose of 1 ml is applied to the affected skin surface twice a day.
The daily dose should not exceed 2 ml, regardless of the size of the treated area.
Frequency of use
Apply 1 ml of solution in the morning and 1 ml of solution in the evening.
Do not increase the dose or frequency of use.
In all cases, the recommended dose should be strictly followed.
Route of administration
For topical use. For external use only.
Before and after applying the solution to the scalp, wash your hands thoroughly.
Before using the medicine, dry the hair and scalp thoroughly.
Apply the medicine with your fingertips, to the entire affected area, starting from the center of the affected area.
Do not use the medicine on any other part of the body.
Instructions for use
Open the bottle: The bottle is equipped with a child-resistant cap.
To open the bottle: press the plastic cap and turn it counterclockwise (to the left). Only the protective ring should remain on the bottle.
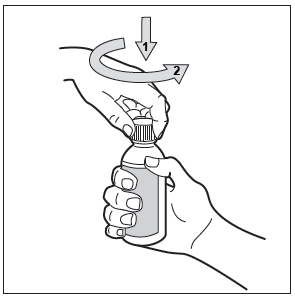
Depending on the type of applicator: dosing pipette or dosing pump with applicator:
Dosing pipette 1 ml
The dosing pipette allows for precise application of 1 ml of solution to the entire affected area, starting from the center of the affected area.
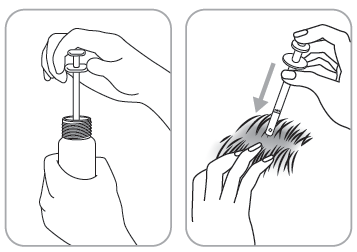
Dosing pump with applicator
The applicator allows for application of the medicine to a small area of skin or under the hair.
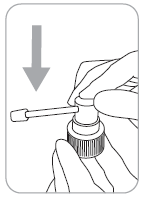
- 1. Attach the applicator to the dosing pump: hold the pump firmly while pressing the top part of the applicator.
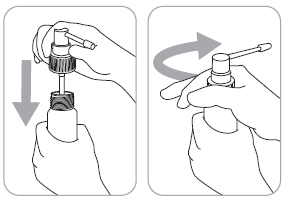
- 2. The applicator connected to the dosing pump is placed in the bottle and tightened firmly.
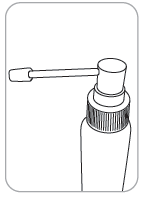
- 3. The pump is ready: the medicine is ready for application.
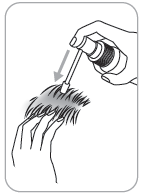
- 4. Application: direct the applicator to the center of the affected skin area or under the hair, press once and spread the medicine with your fingertips.
To apply a dose of 1 ml, 6 presses of the pump are necessary.
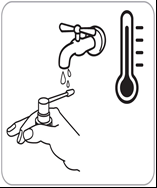
- 5. After each use, rinse the applicator with hot water.
Duration of treatment
The results of treatment are not visible immediately: cessation of hair loss and/or regrowth of hair may be visible after some time from the start of treatment. Continuation of treatment is necessary to stimulate and maintain hair regrowth. The first results of treatment may be visible after 2 to 4 months of using the medicine twice a day. The onset and degree of response to treatment vary from patient to patient.
Some reports suggest that a return to the initial state may be observed after 3 or 4 months of stopping treatment.
Using a higher dose of Alopexy, 50 mg/ml, solution for the skin than recommended
If the medicine is used as recommended, overdose is unlikely.
In case of application to damaged scalp skin, absorption of the active substance may be increased, which may cause side effects (see "2. Important information before using Alopexy, 50 mg/ml, solution for the skin").
Missing a dose of Alopexy, 50 mg/ml, solution for the skin
Do not use a double dose to make up for a missed dose.
Continue using the medicine at the standard frequency: once in the morning and once in the evening.
Stopping treatment with Alopexy, 50 mg/ml, solution for the skin
Not applicable.
If you have any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Consult a doctor immediately if you experience any of the following symptoms - it may be necessary to receive immediate treatment:
- Swelling of the face, lips, or throat, which may cause difficulty in swallowing or breathing. This may indicate a severe allergic reaction (frequency not known, cannot be estimated from the available data).
The most common side effects are mild skin reactions.
Frequent application to the skin may cause irritation and dryness of the skin due to the presence of alcohol in the medicine.
The following side effects have been reported, classified by frequency:
Very common: may occur in more than 1 in 10 people:
Excessive hair growth (excessive hair growth) in areas where the medicine was not applied, especially if the medicine is used in women.
Headaches.
Common: may occur in up to 1 in 10 people:
Local skin reactions at the application site, such as local irritation with scaling (exfoliation), itching, redness of the skin, skin inflammation, dryness of the skin, allergic skin reactions, skin inflammation, acne-like rash, muscle and skeletal pain, peripheral edema (fluid retention in tissues), pain at the application site, breathing difficulties, depression, and pain.
Frequency not known: frequency cannot be estimated from the available data:
Ear infections, external ear inflammation, nasal mucosa inflammation, hypersensitivity, nerve inflammation, tingling sensation, taste disorders, burning sensation of the skin, vision disorders, eye irritation, dizziness, low blood pressure, rapid heartbeat, palpitations, chest pain, weakness, facial edema (fluid retention in tissues), generalized erythema, hair loss, uneven body hair, changes in hair color, changes in hair texture, liver inflammation, and kidney stones.
If you experience any of the above symptoms, stop treatment and consult a doctor immediately.
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, inform your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Alopexy, 50 mg/ml, solution for the skin
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after: EXP.
The expiry date refers to the last day of the month stated.
The medicine is flammable. Keep away from heat sources, hot surfaces, sparks, open flames, and other ignition sources. Do not smoke.
Shelf life after first opening the bottle: 1 month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Alopexy, 50 mg/ml, solution for the skin contains
- The active substance of the medicine is minoxidil. 1 ml of solution contains 50 mg of minoxidil.
- Other ingredients: propylene glycol*, ethanol 96%*, purified water. *(See section 2. "Alopexy, 50 mg/ml, solution for the skin contains propylene glycol and ethanol")
What Alopexy, 50 mg/ml, solution for the skin looks like and what the packaging contains
The medicine is a clear, light yellow solution.
Medicine packaging:
1 or 3 bottles containing 60 ml of solution for the skin, with a child-resistant cap and a dosing pipette and dosing pump with applicator, placed in a cardboard box.
1 bottle containing 60 ml of solution for the skin, with a child-resistant cap and a dosing pipette, placed in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder
Pierre Fabre Medicament
Les Cauquillous
81500 Lavaur
France
Manufacturer
Pierre Fabre Medicament Production
Site PROGIPHARM
Rue du Lycée
45500 Gien
France
This medicine is authorized for marketing in the Member States of the European Economic Area under the following names:
To obtain more detailed information, consult the representative of the marketing authorization holder:
Pierre Fabre Dermo-Cosmetique Polska Sp. z o.o.
ul. Belwederska 20/22
| Austria, Belgium, France, Greece, Luxembourg, Germany, Poland, Romania | Alopexy |
| Italy | Trefostil |
| Portugal | Alorexyl |
00-762 Warsaw
tel. 22 559 63 60
Date of last revision of the leaflet:12/2024
Detailed information about this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
Health education advice What is androgenetic alopecia?
Normally, hair has a lifespan of 3 to 6 years, and 50 to 100 hairs fall out daily.
Excessive hair loss may occur seasonally (in autumn) or after childbirth: this is normal, temporary, and does not require treatment.
Alopecia is defined as hair loss of more than 100 hairs per day.
There are two types of alopecia:
- Acute alopecia,
- Chronic alopecia.
Acute alopecia (sudden hair loss in a short time) may be diffuse or patchy (alopecia) and is most often caused by known causes (aggressive hair care, poor general condition, unbalanced weight loss program, certain medicines, psychological shock, stress, etc.).
Acute alopecia should not be treated with this medicine.
Chronic (long-term) alopecia is almost always diffuse.
Sometimes it is caused by a specific disease (related to the thyroid, metabolism, etc.).
Chronic alopecia should not be treated with this medicine.
In the vast majority of cases, chronic alopecia has a poorly understood origin, most often hereditary: this is androgenetic alopecia. Only this type of alopecia can be treated with minoxidil.
How to recognize androgenetic alopecia in men?
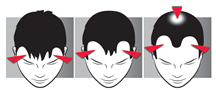
Androgenetic alopecia begins with hair loss in the temporal areas.
Then, a slight baldness appears on the top of the skull.
These two areas without hair will gradually increase: androgenetic alopecia gradually progresses and forms a baldness.
Tips for use
Use a gentle shampoo (preferably without silicones).
If you plan to wash your hair with shampoo after using minoxidil, wait at least 4 hours.
If you use minoxidil after washing your hair with shampoo, it is recommended to apply it to dry scalp skin. After applying minoxidil, you can use a hair dryer, preferably with a warm air flow.
You can use styling foams, gels, or sprays, but wait at least 1 hour between applications.
There are no interactions between minoxidil treatment and permanent waving or coloring, but they should be limited due to their aggressive effect on hair.
If you plan to expose your hair to the sun after using minoxidil, it is recommended to wear a hat.
A few additional tips
At the beginning of treatment, for a short time, there may be an exacerbation of hair loss. These hairs were going to fall out and will fall out faster. This is a normal phenomenon, and treatment can be continued.
The first regrowing hairs will be soft, fluffy, and barely visible. As treatment continues, they may change and become thicker.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterPierre Fabre Medicament Production Site PROGIPHARM
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AlopexiDosage form: Tablets, 1 mgActive substance: finasteridePrescription requiredDosage form: Tablets, 1 mgActive substance: finasteridePrescription requiredDosage form: Tablets, 1 mgActive substance: finasterideManufacturer: mibe GmbH ArzneimittelPrescription required
Alternatives to Alopexi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Alopexi in Spain
Alternative to Alopexi in Ukraine
Online doctors for Alopexi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Alopexi – subject to medical assessment and local rules.







