

Fanhdi

Ask a doctor about a prescription for Fanhdi

How to use Fanhdi
PACKAGE LEAFLET: INFORMATION FOR
THE USER
FANHDI
1000 IU FVIII + 1200 IU VWF
Powder and solvent for solution for injection and infusion
Human coagulation factor VIII and human von Willebrand factor complex
It is essential to read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- The package leaflet should be kept in case it needs to be read again.
- In case of any doubts, the patient should consult a doctor or pharmacist.
- This medicine has been prescribed for a specific person. It should not be given to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this package leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the package leaflet:
- 1. What is FANHDI and what is it used for
- 2. Important information before using FANHDI
- 3. How to use FANHDI
- 4. Possible side effects
- 5. How to store FANHDI
- 6. Contents of the pack and other information
1. WHAT IS FANHDI AND WHAT IS IT USED FOR
FANHDI is a powder and solvent for solution for injection and infusion in vials containing nominally 1000 IU of human coagulation factor VIII (FVIII) and 1200 IU of human von Willebrand factor (VWF). After reconstitution with the provided solvent (water for injection), the product contains 100 IU/ml FVIII and 120 IU/ml VWF. Therapeutic category: antihemorrhagic agents, combination of factor VIII and von Willebrand factor. FANHDI is used to prevent and control bleeding in patients with hemophilia A (congenital factor VIII deficiency).
- 1.3.1. SPC, labelling and package leaflet
The use of FANHDI is also indicated for the prevention and control of bleeding (including surgical bleeding) in patients with von Willebrand disease (VWD) when treatment with desmopressin (DDAVP) is ineffective or contraindicated. The product may be used to treat acquired factor VIII deficiency.
2. IMPORTANT INFORMATION BEFORE USING FANHDI
When not to use FANHDI
- If the patient has a known hypersensitivity (allergy) to the factor VIII/von Willebrand factor complex or to any of the other components of the medicine (listed in section 6).
The patient should consult a doctor if they need advice or additional information.
Warnings and precautions
- Rarely, anaphylactic reactions (severe allergic reactions) may occur. Allergy to FANHDI may manifest as a rash, generalized urticaria, feeling of pressure in the chest, dizziness, even when standing. If these symptoms occur, the administration of the medicine should be stopped and the doctor informed.
- To determine the dose of FANHDI that will achieve and maintain the appropriate level of factor VIII, the doctor may order a series of tests.
- If bleeding does not stop despite the administration of FANHDI, the doctor should be informed. This may be due to the development of a factor VIII inhibitor, which requires confirmation by testing. Factor VIII inhibitors are antibodies that block the action of the administered factor VIII, resulting in a decrease in the effectiveness of factor VIII in stopping bleeding.
- If a factor VIII inhibitor has developed previously and the treatment has been changed to another factor VIII-containing product, there is a higher risk of recurrence of this complication.
- During treatment of von Willebrand disease with known clinical or laboratory risk factors for thrombosis, there is a risk of thrombotic complications. Therefore, it is necessary to perform appropriate tests to detect early signs of thrombosis and to use currently recommended treatments for thrombotic complications.
- In von Willebrand disease, particularly type 3, neutralizing antibodies (inhibitors) against von Willebrand factor may develop. Von Willebrand factor inhibitors are antibodies in the blood that can block the administered factor. In such cases, where the expected levels of von Willebrand factor activity in plasma are not achieved or bleeding is not controlled despite the use of appropriate doses, tests should be performed to detect the presence of von Willebrand factor inhibitors. In patients with high levels of inhibitors, treatment with von Willebrand factor may be ineffective.
- 1.3.1. SPC, labelling and package leaflet
If it is necessary to use a central venous catheter for the administration of FANHDI, the doctor should be aware of the possibility of local infection, bacteremia (blood infection by bacteria), and thrombosis in the vein at the site of the inserted catheter.
- In the manufacturing process of products derived from human blood or plasma, the following measures are taken to ensure safety against the transmission of infectious agents:
- careful selection of donors to exclude carriers of infectious agents,
- testing of each donation and plasma pool for the presence of viruses,
- use of virus inactivation/removal procedures in the manufacturing process.
Despite this, it is not possible to completely exclude the transmission of infectious agents during the use of products derived from human blood or plasma. This also applies to unknown or newly emerging viruses and other pathogens. The methods used are considered effective against enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B (HBV), hepatitis C (HCV), and non-enveloped hepatitis A virus. The effectiveness of these methods against non-enveloped viruses such as parvovirus B19 may be limited. Parvovirus B19 infection can be particularly dangerous for pregnant women (fetal infection) and individuals with impaired immunity or certain types of anemia (e.g., sickle cell anemia or hemolytic anemia). In patients receiving regular repeated doses of plasma-derived products containing factor VIII, the treating doctor may recommend the use of appropriate vaccinations (against hepatitis A and B). It is strongly recommended that when administering FANHDI to a patient, the patient's name and batch number of the product be recorded to enable the patient to be linked to the batch of the medicine. See also section 4.
Children and adolescents
The warnings and precautions mentioned apply to both adults and children.
- 1.3.1. SPC, labelling and package leaflet
FANHDI and other medicines
The patient should inform their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. Interactions between the human factor VIII/von Willebrand factor complex and other medicines are not known.
Pregnancy and breastfeeding
Due to the fact that hemophilia A is rare in women, there is a lack of data on the use of the FVIII/VWF complex during pregnancy and breastfeeding. The patient should consult a doctor or pharmacist before taking any medicine.
Driving and using machines
FANHDI does not affect the ability to drive and use machines.
3. HOW TO USE FANHDI
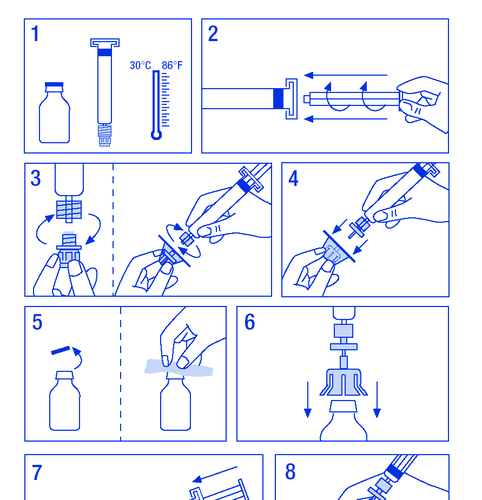
The medicine must be administered intravenously. The rate of administration should not exceed 10 ml/min. The patient should follow the doctor's or healthcare professional's instructions. In case of doubts, the patient should consult a doctor or pharmacist. The dose of FANHDI depends on several factors, such as body weight, clinical condition, and type and severity of bleeding. To achieve the appropriate level of factor VIII and von Willebrand factor in the blood, the doctor will determine the dose of FANHDI and the frequency of administration. The doctor will determine the duration of treatment with FANHDI. The patient should not store the leftovers for later use, even if they are stored in the refrigerator. Preparation of the solution: The patient should ensure that the actions are performed in conditions that prevent contamination.
- 1. Warm the vials to a temperature not exceeding 30°C (Figure 1).
- 2. Attach the plunger to the syringe with the solvent (Figure 2).
- 3. Remove the filter from the packaging. Remove the plastic cap from the end of the syringe and attach the filter (Figure 3).
- 1.3.1. SPC, labelling and package leaflet
- 4. Remove the connector from the vial and connect the syringe with the filter (Figure 4).
- 5. Remove the plastic cap from the vial and expose the rubber stopper, disinfect it with a disinfectant (Figure 5).
- 6. Pierce the stopper in the vial with the needle of the connector (Figure 6).
- 7. Inject the entire solvent into the vial (Figure 7).
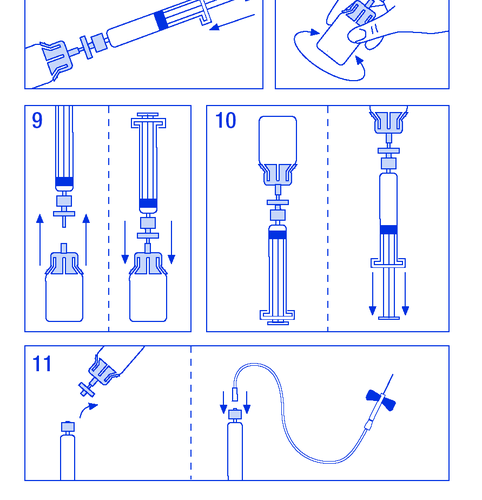
- 8. Gently swirl the vial until the powder is dissolved (Figure 8). As with other intravenous products, do not use if the product is not dissolved or particles are visible.
- 9. Disconnect the syringe with the filter from the vial for a moment to allow air to enter (Figure 9).
- 10. Invert the vial and aspirate the solution into the syringe (Figure 10).
- 11. Prepare the injection site, remove the needle, and inject the product through a butterfly needle or other sterile needle. Administer slowly intravenously at a rate of 3 ml/min and never exceed 10 ml/min to avoid vascular reactions (Figure 11).
- 1.3.1. SPC, labelling and package leaflet
- 1.3.1. SPC, labelling and package leaflet
The administration set should not be used again. Any unused product and other waste should be disposed of in accordance with local regulations.
Use of a higher than recommended dose of FANHDI
No cases of overdose of the human factor VIII/von Willebrand factor complex have been reported. After significant overdose, thrombotic complications may occur. Regardless of this, any case of exceeding the recommended dose of FANHDI should be immediately consulted with a pharmacist or doctor.
Missed dose of FANHDI
- If a dose is missed, it should be administered as soon as possible and the treatment should be continued regularly according to the doctor's recommendations.
- A double dose should not be used to make up for a missed dose.
4. POSSIBLE SIDE EFFECTS
Like all medicines, FANHDI can cause side effects, although not everybody gets them. Allergic reactions or hypersensitivity (vasovagal reaction, burning or stinging at the injection site, chills, flushing, generalized urticaria, headache, rash, hypotension, lethargy, nausea, anxiety, tachycardia, feeling of pressure in the chest, dizziness, vomiting, sweating) have been observed rarely and only in some cases led to the development of severe anaphylaxis (including shock). In rare cases, an increase in body temperature has been observed. In case of anaphylactic or allergic reaction, the administration of the medicine should be stopped and the doctor informed immediately. It is not possible to completely exclude the possibility of allergic reactions after the administration of this medicine. Patients with hemophilia A may develop neutralizing antibodies (inhibitors) against factor VIII. In case of the development of such inhibitors, an insufficient clinical response to treatment is observed. In very rare cases, patients with von Willebrand disease, particularly type 3, may develop neutralizing antibodies (inhibitors) against von Willebrand factor. If such inhibitors develop, an insufficient clinical response to treatment is observed. Inhibitors may increase the risk of allergic reactions (anaphylactic shock). In case of allergic reactions, tests for the presence of inhibitors should be performed. In such cases, it is recommended to contact a specialized hemostasis treatment center. During the use of FANHDI in patients with von Willebrand disease with known clinical or laboratory risk factors for thrombosis, there is a risk of thrombotic complications. Maintaining high levels of FVIII during treatment with factor VIII-containing products increases the risk of thrombotic complications. During several clinical trials involving 164 patients, a total of 7000 infusions of FANHDI were administered. The results of both studies indicate good tolerance of the medicine and a low frequency of side effects. Only two cases of side effects related to the administered product were observed, in which an increase in body temperature was noted. Table of side effects The following table lists the system organ classes and preferred terms (MedDRA classification). The frequency of side effects is classified according to the following criteria:
- very common (≥ 1/10)
- common (≥ 1/100 to <1>
- uncommon (≥ 1/1000 to <1>
- rare (≥ 1/10,000 to <1>
- very rare (<1>
- not known (frequency cannot be estimated from the available data).
In each frequency category, side effects are listed in order of severity, from most severe to least severe.
| System organ class | Side effects | Frequency |
| General disorders and administration site conditions | Increased body temperature | Rare |
- 1.3.1. SPC, labelling and package leaflet
Children and adolescents
The frequency, type, and severity of side effects expected in children do not differ from those observed in adults.
Reporting of suspected side effects
After the authorization of the medicine, it is essential to report any suspected side effects. This allows for the continuous monitoring of the benefit-risk balance of the medicine. Healthcare professionals should report any suspected side effects via the national reporting system:
Department for the Monitoring of Adverse Reactions to Medicinal Products, Medical Devices, and Biocidal Products, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, e-mail: [email protected]. Reporting side effects helps to gather more information on the safety of the medicine.
5. HOW TO STORE FANHDI
The medicine should be stored out of the sight and reach of children. Do not store above 30°C. Do not freeze. The medicine should not be used after the expiry date. The solution should be clear and slightly opalescent. Do not use solutions that contain particles or sediment. Do not use if the product contains particles or the solution has changed color after reconstitution. After reconstitution, the chemical and physical stability of the product is maintained for 12 hours at 25°C. From a microbiological point of view, the product should be used immediately. If the product is not used after reconstitution, it can be stored for no longer than 24 hours at 2-8°C, but only if the user is responsible for preparing the solution in accordance with the principles of asepsis. Medicines should not be disposed of via wastewater. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
Shelf life
3 years. The medicine should not be used after the expiry date stated on the label.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What FANHDI contains
The active substance is human coagulation factor VIII and human von Willebrand factor complex. Each vial of powder contains 1000 IU of human coagulation factor VIII and 1200 IU of von Willebrand factor. After reconstitution with 10 ml of water for injection, the product contains 100 IU/ml FVIII and 120 IU/ml VWF. The other ingredients are human albumin, histidine, and arginine. Each syringe contains 10 ml of water for injection.
What FANHDI looks like and contents of the pack
Vial with white or light yellow powder and syringe with water for injection. Each pack of FANHDI contains a vial of 1000 IU of human coagulation factor VIII and 1200 IU of von Willebrand factor (powder for injection and infusion) and 1 syringe of 10 ml water for injection (solvent). The preparation and administration set is included in the pack: connector for the vial, filter, and infusion set. Available packs: FANHDI 250 IU FVIII + 300 IU VWF, FANHDI 500 IU FVIII + 600 IU VWF, FANHDI 1000 IU FVIII + 1200 IU VWF. Not all packs may be marketed.
- 1.3.1. SPC, labelling and package leaflet
Marketing authorization holder and manufacturer
Instituto Grifols, S.A., Can Guasc, 2 - Parets del Vallès, 08150 Barcelona, Spain. For more information, the patient should contact their local representative of the marketing authorization holder: Grifols Polska Sp. z o.o., Ul. Grzybowska 87, 00-844 Warszawa, Tel: +48 22 378 85 61.
Date of last revision of the package leaflet:
…………………………………………………………………………………………………
Information intended for healthcare professionals only:
Dosage
Factor VIII deficiency. The dosage and duration of substitution therapy depend on the severity of the factor VIII deficiency, the location and extent of the bleeding, and the patient's clinical condition. The administered dose of factor VIII is expressed in international units (IU) in accordance with the current WHO standards for factor VIII products. The activity of factor VIII in plasma can be expressed as a percentage of normal activity or in IU/ml. One international unit (IU) of factor VIII activity is equivalent to the amount of factor VIII in 1 ml of normal human plasma.
Calculation of the required dose
The required dose is based on empirical observations that the administration of 1 IU of factor VIII per kg of body weight increases the activity of factor VIII in plasma by 1.7% to 2.5% of normal activity. The dose is calculated using the following formula:
Required dose (IU) = body weight (kg) x desired increase in factor VIII activity (%) x 0.5
The dose and frequency of administration should always be adjusted individually for each patient, depending on their response to treatment. In the treatment of bleeding, depending on the cause and location, it is necessary to maintain the appropriate level of factor VIII activity (as a percentage of normal or IU/ml) for the recommended duration of treatment. When determining the dose based on the type of bleeding or surgical procedure, the following table can be used:
| Severity of bleeding/surgical procedure | Required factor VIII level (%) (IU/ml) | Frequency of dosing (hours)/duration of treatment (days) |
| Bleeding: Mild bleeding into joints, muscles, or oral bleeding. More severe bleeding into joints, muscles, or hematoma. Life-threatening bleeding. |
| Repeat every 12-24 hours for at least 1 day, until pain caused by bleeding subsides or wound healing. Repeat infusions every 12-24 hours for 3-4 days or longer, until pain or functional impairment subsides. Repeat infusions every 8-24 hours until the risk of bleeding has subsided. |
| Surgical procedures: Minor, including tooth extraction Major |
| Every 24 hours, for at least 1 day, until wound healing. Repeat infusions every 8-24 hours until adequate wound healing is achieved, then continue treatment for another 7 days, maintaining factor VIII activity at 30% to 60% (IU/ml). |
Prophylactic treatment. In long-term prophylaxis of bleeding in patients with severe hemophilia A, a dose of 20 to 40 IU/kg body weight is usually used at intervals of 2 to 3 days. In some cases, especially in young patients, it may be necessary to shorten the intervals between injections or increase the dose. During treatment, to determine the dose and frequency of infusions, it is recommended to monitor the levels of factor VIII in plasma. Especially in cases of major surgical procedures, it is necessary to closely monitor substitution therapy using coagulation tests (factor VIII activity in plasma). Patients may respond individually to factor VIII treatment, which is reflected in different in vivo recovery levels and half-lives in individual patients. Von Willebrand disease. Generally, it is assumed that the administration of 1 IU of VWF:RCo per kg of body weight increases the level of VWF:RCo by 2% in the circulation. The goal of treatment is to achieve a level of VWF:RCo > 0.6 IU/ml (60%) and FVIII:C > 0.4 IU/ml (40%) in plasma. In most cases, to achieve hemostasis, a dose of 40-80 IU/kg body weight of von Willebrand factor and 20-40 IU/kg body weight of factor VIII:C is recommended. Patients with type 3 von Willebrand disease, who may require higher doses to maintain adequate levels of factor, may need an initial dose of 80 IU/kg body weight of von Willebrand factor. The appropriate dose should be administered every 12-24 hours. The dosage and duration of treatment depend on the patient's clinical condition, the location and extent of bleeding, and the levels of both VWF:RCo and FVIII:C. During the use of factor VIII products containing von Willebrand factor, the treating doctor should consider the possibility of excessive increase in FVIII:C levels. To avoid excessive increase in FVIII:C levels, after 24-48 hours of treatment, it is recommended to consider reducing the dose or prolonging the interval between doses or using products containing VWF with lower factor VIII content. Children and adolescents. In the above-mentioned indications, only limited data from clinical trials are available for children under 6 years of age, and therefore, there are no recommendations for the use of the product in this age group. In children, as in adults, the dose should be adjusted based on clinical efficacy, taking into account the patient's body weight.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterInstituto Grifols, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FanhdiDosage form: Powder, 50 IU/ml; 500 IU + 60 IU/ml; 600 IUActive substance: von Willebrand factor and coagulation factor VIII in combinationManufacturer: Instituto Grifols S.A.Prescription requiredDosage form: Powder, 25 IU/ml; 250 IU + 30 IU/ml; 300 IUActive substance: von Willebrand factor and coagulation factor VIII in combinationManufacturer: Instituto Grifols S.A.Prescription requiredDosage form: Powder, 1000 IU + 2400 IU/15 mlActive substance: von Willebrand factor and coagulation factor VIII in combinationManufacturer: CSL Behring GmbHPrescription required
Alternatives to Fanhdi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fanhdi in Ukraine
Alternative to Fanhdi in Spain
Online doctors for Fanhdi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fanhdi – subject to medical assessment and local rules.














