
Exacil

Ask a doctor about a prescription for Exacil

How to use Exacil
Package Leaflet: Information for the User
EXACYL, 1 g/10 ml, Oral Solution
Tranexamic Acid
Read the package leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Exacyl and what is it used for
- 2. Important information before taking Exacyl
- 3. How to take Exacyl
- 4. Possible side effects
- 5. How to store Exacyl
- 6. Contents of the pack and other information
1. What is Exacyl and what is it used for
Exacyl contains the active substance tranexamic acid. Tranexamic acid belongs to a group of
medicines called antifibrinolytics. The action of tranexamic acid is to inhibit the fibrinolytic activity of plasmin.
Exacyl is used in:
- bleeding from the reproductive tract: caused by hormonal disorders or occurring secondary to injuries, infections, or degenerative changes in the uterus;
- bleeding from the gastrointestinal tract;
- hematuria from the lower urinary tract caused by: benign prostatic hyperplasia, malignant tumors of the prostate and bladder, kidney stones, or bleeding from the urinary tract after surgical procedures on the prostate and urinary system;
- bleeding associated with otolaryngological surgical procedures (e.g., tonsillectomy).
2. Important information before taking Exacyl
When not to take Exacyl
Warnings and precautions
Before starting treatment with Exacyl, discuss it with your doctor or pharmacist:
You should immediately inform your doctor if you experience any of the following symptoms:
unusual leg pain, muscle weakness, chest pain, irregular pulse, sudden shortness of breath, loss of consciousness, disorientation, severe headache, dizziness, visual disturbances, slowed speech or loss of speech.
Exacyl and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have recently taken, as well as any medicines you plan to take, including those available without a prescription.
In particular, inform your doctor if you are taking:
- other medicines that facilitate blood clotting, called antifibrinolytic agents (treatment should be carried out under close medical supervision)
- medicines that prevent blood clotting, called thrombolytic agents
- oral contraceptives.
You should also inform your doctor if you are taking:
- Etamsylate (a medicine that stops bleeding)
- Vitamin K1 and tiemonium methylsulfate (an antispasmodic medicine)
Exacyl with food and drink
Exacyl can be taken with or without food.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before taking this medicine.
Avoid taking this medicine during pregnancy.
Tranexamic acid passes into breast milk. Breastfeeding is not recommended during treatment with this medicine.
Driving and using machines
During treatment with Exacyl, dizziness and malaise may occur. If you experience such symptoms, do not drive or operate machinery.
Exacyl contains alcohol and sodium
This medicine contains a maximum of 4.9 mg of alcohol (ethanol) per ml, which is equivalent to 0.62% by volume. The amount of alcohol in 20 ml (the maximum single dose) of this medicine is equivalent to less than 3 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine will not have noticeable effects.
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means that the medicine is considered "sodium-free".
3. How to take Exacyl
Take this medicine always as directed by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
The recommended dose is:
Adults
2 to 4 g per day in 2 or 3 divided doses (i.e., 2 to 4 ampoules per day).
Children
20 mg/kg body weight per day.
The solution should be taken orally.
How to open the ampoules:
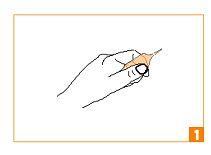 | Hold the ampoule in your hand, between your thumb and index finger, as shown. |
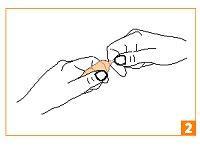 | Grasp the end of the ampoule between your thumb and index finger of your other hand. |
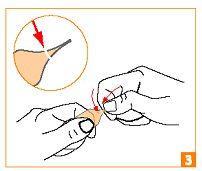 | Gently press your thumb on the ring indicating the break point, while providing resistance with your index finger of your other hand. Do not twist. |
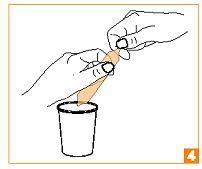 | Repeat the entire procedure to open the other end, being careful to hold the open end over a glass. |
Patients with renal impairment
In the case of renal impairment, due to the risk of accumulation of the medicinal product, the dosage of tranexamic acid should be reduced according to the serum creatinine concentration.
The following dosing schedule applies to EXACYL, solution for injection, and should only be used for the solution for injection.
If the serum creatinine concentration is:
between 120 and 250 μmol/l, the dose of tranexamic acid is 10 mg/kg body weight intravenously, twice a day;
between 250 and 500 μmol/l, the dose of tranexamic acid is 10 mg/kg body weight intravenously, once a day (every 24 hours);
above 500 μmol/l, the dose of tranexamic acid is 5 mg/kg body weight intravenously, once a day (every 24 hours).
Overdose of Exacyl
In the event of an overdose, the following may occur: dizziness of central origin, headache, hypotension, and seizures. Seizures tend to occur more frequently with increasing doses. If you have taken more than the recommended dose of Exacyl, seek medical attention immediately.
Missed dose of Exacyl
If you miss a dose, take it as soon as possible, unless it is almost time for your next dose.
Do not take a double dose to make up for a missed dose.
Stopping treatment with Exacyl
If you have any further questions about taking this medicine, ask your doctor or pharmacist.
4.
Possible side effects
Like all medicines, Exacyl can cause side effects, although not everybody gets them.
Side effects reported during treatment with Exacyl.
During treatment with Exacyl, the following side effects have been observed:
Common(may affect up to 1 in 10 people):
- nausea, vomiting, diarrhea.
Uncommon(may affect up to 1 in 100 people):
- skin allergic reactions.
Frequency not known(frequency cannot be estimated from the available data):
- hypersensitivity reactions, including anaphylaxis (a severe and sudden allergic reaction);
- seizures, especially in the presence of risk factors or a history of seizures, or also in the case of improper use of the medicine (see section 2);
- visual disturbances, including changes in color vision;
- malaise with hypotension, with or without loss of consciousness (usually after too rapid intravenous injection, exceptionally after oral administration);
- venous or arterial thrombosis in different parts of the body;
- fixed drug eruption;
- sudden renal impairment.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: + 48 22 49 21 301, Fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative in Poland.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Exacyl
Keep this medicine out of the sight and reach of children.
Store in a temperature below 25°C, protected from light.
Do not use this medicine after the expiry date stated on the carton. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Exacyl contains
- The active substance is tranexamic acid. 1 ml of oral solution contains 100 mg of tranexamic acid. 1 ampoule (10 ml) contains 1 g of tranexamic acid.
- The other ingredients are: cherry flavor, hydrochloric acid or sodium hydroxide for pH adjustment, purified water.
What Exacyl looks like and contents of the pack
Ampoules placed in a cardboard box.
The pack contains 5 ampoules of 10 ml each.
Marketing authorization holder
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Germany
Manufacturer
Unither Liquid Manufacturing
1-3 Alèe de la Neste
ZI d’en Sigal
31770 Colomiers, France
Cooperation Pharmaceutique Francaise
Place Lucien Auvert
77020 Melun, France
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterCooperation Pharmaceutique Francaise Unither Liquid Manufacturing
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ExacilDosage form: Tablets, 500 mgActive substance: tranexamic acidPrescription requiredDosage form: Tablets, 1000 mgActive substance: tranexamic acidManufacturer: Adamed Pharma S.A.Prescription requiredDosage form: Solution, 100 mg/mlActive substance: tranexamic acidManufacturer: Delpharm Dijon Sanofi Winthrop IndustriePrescription required
Alternatives to Exacil in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Exacil in Spain
Alternative to Exacil in Ukraine
Online doctors for Exacil
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Exacil – subject to medical assessment and local rules.














