
AMCHAFIBRIN 500 mg INJECTABLE SOLUTION

How to use AMCHAFIBRIN 500 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
Amchafibrin 500 mg solution for injection
tranexamic acid
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What Amchafibrin 500 mg solution for injection is and what it is used for
- What you need to know before you use Amchafibrin 500 mg solution for injection
- How to use Amchafibrin 500 mg solution for injection
- Possible side effects
- Storage of Amchafibrin 500 mg solution for injection
- Contents of the pack and other information
1. What Amchafibrin 500 mg solution for injection is and what it is used for
Amchafibrin contains tranexamic acid, which belongs to a group of medicines called antihemorrhagics, antifibrinolytics, amino acids.
Amchafibrin is used in adults and children over one year of age for the prevention and treatment of blood loss due to a process that inhibits blood coagulation called fibrinolysis.
The specific indications include:
- Heavy menstrual bleeding in women
- Gastrointestinal bleeding
- Hemorrhagic disorders of the urinary tract, after prostate surgery or surgical procedures that affect the urinary tract
- Ear, nose, and throat surgery
- Heart, abdominal, or gynecological surgery
- Bleeding after being treated with another medicine to dissolve blood clots
2. What you need to know before you use Amchafibrin 500 mg solution for injection
Do not useAmchafibrin 500 mgif:
- You are allergic to tranexamic acid or any of the other components of this medicine (listed in section 6).
- You currently have a disease that causes the formation of blood clots
- You have a problem called 'consumption coagulopathy' in which the blood throughout the body begins to clot
- You have kidney problems
- You have a history of seizures
Due to the risk of cerebral edema and seizures, intrathecal and intraventricular injection and intracerebral application are not recommended.
If you think any of the above circumstances apply to you or you have any doubts, inform your doctor before taking Amchafibrin 500 mg.
Warnings and precautions
Consult your doctor if any of these circumstances apply to you before starting to use Amchafibrin 500 mg:
- If you have blood in your urine, Amchafibrin 500 mg may cause obstruction of the urinary tract.
- If you are at risk of forming blood clots.
- If you have an excess of clot formation or bleeding in the body (disseminated intravascular coagulation), Amchafibrin 500 mg may not be suitable for you, unless you have a severe acute hemorrhage and blood tests show that the process that inhibits blood coagulation called fibrinolysis has been activated.
- If you have had seizures, Amchafibrin 500 mg should not be administered. Your doctor should use the lowest possible dose to avoid seizures after treatment with Amchafibrin 500 mg.
- If you are on long-term treatment with Amchafibrin 500 mg, attention should be paid to possible alterations in color vision, and if necessary, treatment should be suspended. With the long-term continuous use of Amchafibrin 500 mg solution for injection, it is indicated to perform periodic ophthalmological examinations (eye exams such as visual acuity, color vision, fundus study, visual field, etc.). In the event of pathological ophthalmic changes, especially retinal diseases, your doctor should decide, after consulting with a specialist, on the need for long-term use of the Amchafibrin 500 mg solution for injection in your case.
Other medicines andAmchafibrin 500 mg
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription, vitamins, minerals, herbal products, or dietary supplements.
In particular, you should inform your doctor if you are taking:
- other medicines that help blood to clot, called antifibrinolytic medicines
- medicines that prevent blood coagulation, called thrombolytic medicines
- oral contraceptives. They may increase the risk of blood clots.
Pregnancy andbreast-feeding
If you are pregnant or breast-feeding, consult your doctor before using Amchafibrin 500 mg.
Tranexamic acid is excreted in human milk. Therefore, the use of Amchafibrin 500 mg is not recommended during breast-feeding.
Driving and using machines
No studies have been performed on the ability to drive and use machines.
3. How to use Amchafibrin 500mg
Use in adults
Amchafibrin will be administered to you by slow injection into a vein.
Your doctor will decide the correct dose for you and for how long you should receive it.
Use in children
If Amchafibrin is administered to a child over one year of age, the dose should be based on the child's weight. Your doctor will decide the correct dose for the child and for how long they should receive it.
Use in elderly patients
No dose reduction is necessary unless there is evidence of renal insufficiency.
Use in patients with kidney problems
If you have kidney problems, the dose of tranexamic acid should be reduced according to the results of a blood test (serum creatinine level).
Use in patients with liver failure
If you have liver problems, no dose reduction is necessary.
Method of administration
Amchafibrin should only be administered as a slow intravenous injection.
Amchafibrin should not be injected into a muscle.
If you receive more Amchafibrin 500 mg than you should
If you have received a higher dose of Amchafibrin than recommended, you may experience a temporary drop in blood pressure. Talk to a doctor or pharmacist immediately.
You can also contact the Toxicology Information Service. Telephone 91 562 04 20, indicating the medicine and the amount administered.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed with Amchafibrin 500 mg:
Common (may affect up to 1 in 10 patients)
- Gastrointestinal effects: nausea, vomiting, diarrhea
Uncommon (may affect up to 1 in 100 patients)
- Skin effects: rash
Frequency not known (frequency cannot be estimated from the available data)
- Discomfort with hypotension (low blood pressure), especially after a too rapid intravenous injection
- Blood clots
- Nervous system effects: seizures
- Eye effects: vision disorders including color vision impairment
- Immune system effects: allergic reactions
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Amchafibrin 500 mg
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Store in the original package.
Do not use Amchafibrin 500 mg solution for injection after the expiry date which is stated on the carton after “EXP”. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition ofAmchafibrin 500 mgsolution for injection
- The active substance is tranexamic acid. Each ampoule contains 500 mg of tranexamic acid.
- The other ingredients are: water for injections.
AppearanceAmchafibrin 500 mgsolution for injectionand pack contents
Solution for injection. Pack containing 6 and 100 glass ampoules of 5 ml.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublin 15
Dublin
Ireland
Manufacturer
Biologici Italia Laboratories S.R.L
Via Filippo Serpero,
20060 Masate (MI)
Italy
You can obtain further information on this medicine by contacting the local representative of the marketing authorisation holder:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
Spain
Date of last revision of this leaflet:June 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) (https://www.aemps.gob.es/)
The following information is intended for healthcare professionals only:
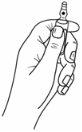
The ampoules have a One Point Cut (OPC) opening system and should be broken according to the following instructions:
- Hold the lower part of the ampoule with your hand, with your thumb pointing to the colored point, as shown below:
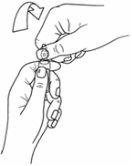
- Hold the upper part of the ampoule with your other hand, supporting your thumb on the colored point and applying pressure in the direction opposite to the colored point, as shown below:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMCHAFIBRIN 500 mg INJECTABLE SOLUTIONDosage form: TABLET, 500 mgActive substance: tranexamic acidManufacturer: Apc Instytut Sp. Z O.O.Prescription requiredDosage form: INJECTABLE, 100 mg/mlActive substance: tranexamic acidManufacturer: Baxter Holding B.V.Prescription requiredDosage form: TABLET, 500 mgActive substance: tranexamic acidManufacturer: Zentiva K.S.Prescription required
Online doctors for AMCHAFIBRIN 500 mg INJECTABLE SOLUTION
Discuss questions about AMCHAFIBRIN 500 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















