
Evertas
Ask a doctor about a prescription for Evertas

How to use Evertas
Leaflet attached to the packaging: information for the user
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Evertas, 9.5 mg/24 h, transdermal system, patch
Rivastigmine
The leaflet should be read carefully before using the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be re-read if necessary.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their disease are the same.
- If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Evertas and what is it used for
- 2. Important information before using Evertas
- 3. How to use Evertas
- 4. Possible side effects
- 5. How to store Evertas
- 6. Package contents and other information
1. What is Evertas and what is it used for
The active substance of Evertas is rivastigmine.
Rivastigmine belongs to a group of substances called cholinesterase inhibitors. In patients with Alzheimer's disease, there is a decrease in the number of nerve cells in the brain, which reduces the concentration of acetylcholine produced by them, a neurotransmitter (a substance that enables communication between nerve cells). The action of rivastigmine involves blocking the enzymes that break down acetylcholine: acetylcholinesterase and butyrylcholinesterase. By blocking the action of these enzymes, rivastigmine allows the concentration of acetylcholine in the brain to increase, which helps to alleviate the symptoms of Alzheimer's disease.
Evertas is used to treat adult patients with mild to moderately severe Alzheimer's disease, a progressive brain disease that gradually disrupts memory, intellectual abilities, and behavior.
2. Important information before using Evertas
When not to use Evertas
- if the patient is allergic to rivastigmine or any of the other ingredients of this medicine (listed in section 6);
- if the patient has an allergic reaction to similar medicines (carbamate derivatives);
- if the patient has a skin reaction that extends beyond the skin area covered by the patch, if the local reaction worsens (e.g., blisters, exacerbation of skin inflammation, swelling) and if these changes do not resolve within 48 hours after removing the patch. If this situation applies to the patient, they should inform their doctor and not apply the Evertas patch.
Warnings and precautions
Before starting to use Evertas, the patient should discuss it with their doctor or pharmacist if:
- the patient has or has had an irregular or slow heart rhythm;
- the patient has or has had an active stomach ulcer;
- the patient has or has had difficulty urinating;
- the patient has or has had seizures;
- the patient has or has had asthma or severe respiratory disease;
- the patient has muscle tremors;
- the patient has a low body weight;
- the patient has gastrointestinal reactions, such as nausea (vomiting), vomiting, and diarrhea. The patient may become dehydrated (lose too much fluid) if vomiting or diarrhea persists for a longer period;
- the patient has liver function disorders. If any of these situations apply to the patient, closer monitoring by the doctor may be necessary during treatment with this medicine.
If the patient has not applied a patch for more than three days, they should not apply a new patch until they have talked to their doctor.
Children and adolescents
The use of Evertas in children and adolescents for the treatment of Alzheimer's disease is not appropriate.
Evertas and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Evertas may affect the action of anticholinergic medicines, some of which are used to relieve stomach cramps (e.g., dicyclomine), to treat Parkinson's disease (e.g., amantadine), or to prevent motion sickness (e.g., diphenhydramine, scopolamine, or meclizine).
Evertas transdermal system, patch should not be used at the same time as metoclopramide (a medicine used to relieve or prevent nausea and vomiting). Taking these two medicines together may cause disorders such as stiffness of the limbs and hand tremors.
If the patient is to undergo surgery during treatment with Evertas transdermal systems, patches, they should tell their doctor about their use, as they may enhance the action of certain muscle relaxants given during anesthesia.
Care should be taken when Evertas transdermal system, patch is used with beta-adrenergic blockers (medicines such as atenolol, used to treat high blood pressure, angina, and other heart diseases). Taking these two medicines together may cause disorders such as slow heart rate (bradycardia) leading to fainting or loss of consciousness.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
If the patient is pregnant, the benefits of using Evertas should be weighed against the possible effects of the medicine on the unborn child. Evertas should not be used during pregnancy unless it is absolutely necessary.
During treatment with Evertas transdermal systems, patches, the patient should not breastfeed.
Driving and using machines
The doctor will inform the patient whether their condition allows them to drive or use machines safely. Evertas (patches) may cause fainting or severe confusion. If the patient feels weak or is disoriented, they should not drive, use machines, or perform other tasks that require concentration.
3. How to use Evertas
This medicine should always be used exactly as described in the patient information leaflet or as directed by the doctor. In case of doubt, the doctor or pharmacist should be consulted.
Evertas is available in doses of 4.6 mg/24 h and 9.5 mg/24 h.
WARNING:
- -Before applying a new patch, the old patch should be removed.
- -Only ONE Evertas patch should be applied per day.
- -The patch should not be cut into smaller pieces.
- -The patch should be pressed firmly onto the skin with the palm of the hand for at least
30 seconds.
How to start treatment
The doctor will inform the patient which Evertas patches are best for their case.
- Treatment usually starts with Evertas 4.6 mg/24 h.
- The recommended daily dose of Evertas is usually 9.5 mg/24 h. If the treatment is well tolerated, the doctor may consider increasing the dose to 13.3 mg/24 h (this dose cannot be achieved with this product. In cases where such a dose is required, other rivastigmine products with 13.3 mg/24 h transdermal systems should be used).
- Only one Evertas patch should be applied at a time, and it should be changed every 24 hours. During treatment, the doctor may adjust the dose of the medicine to the individual patient's needs.
If the patient has not applied a patch for more than three days, they should not apply a new patch until they have talked to their doctor. Treatment can be resumed using the same dose if the interruption in treatment does not exceed three days. Otherwise, the doctor will recommend resuming treatment with a dose of 4.6 mg/24 h Evertas.
Evertas can be used with food, drink, and alcohol.
Where the patient should apply the Evertas patch
- Before applying the patch, the patient should make sure the skin in the area where the patch will be applied is clean, dry, and hairless, free from powder, oils, creams, or liquids that could prevent the patch from sticking properly, and free from cuts, rashes, and/or irritations.
- The patient should carefully remove any previously applied patches before applying a new one. Applying multiple patches to the skin may expose the patient to an excessive amount of medicine, which can be dangerous.
- The patient should apply ONLY ONE patch per day to ONLY ONE of the possible locations, as shown in the diagrams:
- upper part of the left arm orupper part of the right arm
- upper part of the chest on the left side oron the right side ( the patient should avoid applying patches to the breasts)
- upper part of the back on the left side oron the right side
- lower part of the back on the left side oron the right side
After 24 hours, the old patch should be removed before applying a NEW patch to ONLY ONE of the shown locations.
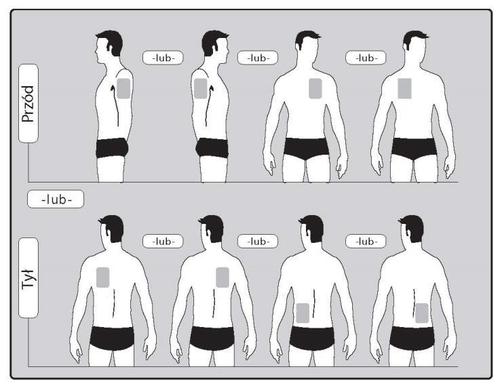
When changing the patch, the patient should remove the previous day's patch before applying a new patch to a different location (e.g., one day on the right side of the body, the next day on the left, one day on the upper part of the body, the next day on the lower part). The patient should not apply a patch to the same location before 14 days have passed.
How the patient should apply the Evertas patches
Evertas is a thin, light-brown, plastic patch that is applied to the skin. Each patch is in a sealed protective sachet. The sachet should not be opened or the patch removed until it is to be applied to the skin.
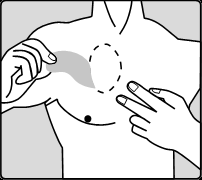
The patient should carefully remove the old patch before applying a new one.
Patient's starting treatment for the first time and patients restarting treatment with rivastigmine after an interruption should start with the actions shown in the second diagram.
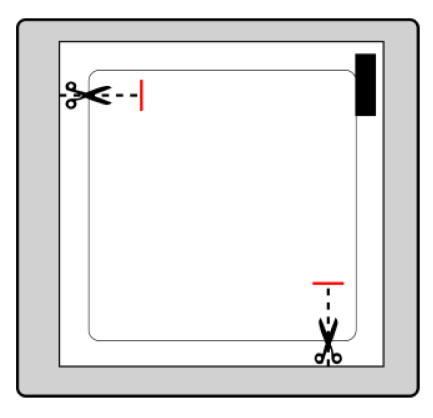
Each patch is in a sealed protective sachet. The sachet should be opened just before using the patch.
The sachet should be cut at both marked locations with scissors, but no further than the indicated line. The sachet should be pierced to open it. The sachet should not be cut along its entire length to avoid damaging the patch.
The patch should be removed from the sachet.
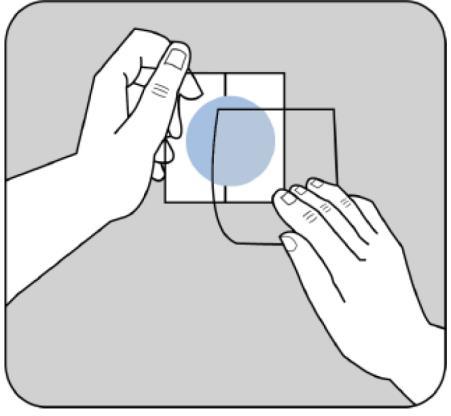
The protective backing should be removed from the upper, beige-colored side of the patch.
The adhesive side of the patch is protected by a protective layer.
The patient should remove one part of the protective layer without touching the adhesive side of the patch.
The adhesive side of the patch should be applied to the upper or lower part of the back, the upper part of the arm, or the chest, and then the second part of the protective layer should be removed.
The patch should be pressed firmly onto the skin with the palm of the hand for at least 30 seconds, making sure the edges are well stuck to the skin.
The patch can now be signed with a pen, e.g., with the name of the day of the week.
The patch should be worn continuously until it is changed for a new one. The patient can try different locations for applying a new patch to find the most convenient and least exposed to abrasion by clothing.
How the patient should remove the Evertas patch
The patch should be gently pulled off from one edge and slowly peeled off the skin. If any adhesive residue remains on the skin, it can be removed by washing the area with warm water and mild soap or baby oil. The patient should not use alcohol or other solvents (nail polish remover or other products).
After removing the patch, the patient should wash their hands with soap and water. If the medicine comes into contact with the eyes or if the eyes become red after contact with the patch, they should be rinsed immediately with plenty of water, and if the symptoms do not resolve, the patient should consult their doctor.
Can the patient use Evertas patches while bathing, swimming, or in the sun?
- Bathing, swimming, or showering should not affect the action of the patch.
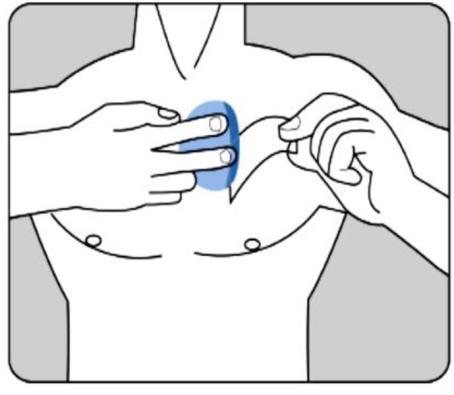
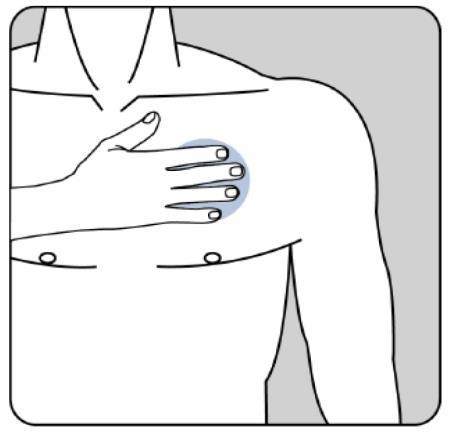
The patient should make sure that the patch does not come loose during these activities.
- The patch should not be exposed to external heat sources (e.g., excessive sun exposure, sauna, solarium) for a longer period.
What to do if the patch comes off
If the patch comes off, a new one should be applied for the rest of the day, and then changed at the usual time the next day.
When and for how long the patient should use Evertas patches
- To get the effect of the treatment, a new patch should be applied every day, preferably at the same time every day.
- Only one Evertas patch should be applied at a time, and it should be changed every 24 hours.
Using a higher dose of Evertas than recommended
If the patient accidentally applies more than one patch, they should remove all patches and inform their doctor about the accidental application of more than one patch. The patient may need medical attention. In some people who have accidentally taken too much rivastigmine, nausea, vomiting, diarrhea, high blood pressure, and hallucinations have occurred. There may also be slow heart rate and fainting.
Missing a dose of Evertas
If the patient realizes they have forgotten to apply a patch, they should do so as soon as possible. The next patch can be applied at the usual time the next day. The patient should not apply two patches to make up for a missed dose.
Stopping treatment with Evertas
If treatment is interrupted, the doctor or pharmacist should be informed.
In case of any further doubts about the use of this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, Evertas can cause side effects, although not everybody gets them.
Side effects occur more frequently during the initial period of treatment or when the dose is increased. Side effects usually resolve slowly as the body adapts to the treatment.
If one of the following side effects occurs, the patch should be removed and the doctor informed immediately, as these effects can be severe:
Common(occurring in less than 1 in 10 patients):
- loss of appetite;
- dizziness;
- agitation or drowsiness;
- urinary incontinence (inability to properly retain urine).
Uncommon(occurring in less than 1 in 100 patients):
- heart rhythm disorders, such as slow heart rate;
- hallucinations;
- stomach ulcers;
- dehydration (loss of too much fluid);
- excessive restlessness (high level of activity, agitation);
- aggression.
Rare(occurring in less than 1 in 1,000 patients):
- falls.
Very rare(occurring in less than 1 in 10,000 patients):
- stiffness of the arms or legs;
- tremors of the hands.
Frequency not known(cannot be estimated from the available data):
- allergic reaction at the patch application site, such as blisters or skin inflammation;
- worsening of Parkinson's disease symptoms - such as tremors, stiffness, or dragging of the legs;
- pancreatitis - symptoms include severe abdominal pain, often with nausea (vomiting) or vomiting;
- rapid or irregular heartbeat;
- high blood pressure;
- seizures (convulsions);
- liver function disorders (jaundice, yellowing of the whites of the eyes, unexplained dark urine, or nausea, vomiting, fatigue, and loss of appetite);
- changes in liver function test results;
- anxiety,
- nightmares.
If one of the above side effects occurs, the patch should be removed and the doctor informed immediately.
Other side effects reported after using rivastigmine in capsule or oral solution form, which may also occur after using patches:
Common(occurring in less than 1 in 10 patients):
- excessive salivation;
- loss of appetite;
- anxiety;
- general feeling of being unwell;
- tremors or confusion;
- excessive sweating.
Uncommon(occurring in less than 1 in 100 patients):
- heart rhythm disorders (e.g., rapid heartbeat);
- difficulty sleeping;
- accidental falls.
Rare(occurring in less than 1 in 1,000 patients):
- seizures (convulsions);
- intestinal ulcers;
- chest pain - may be caused by a heart attack.
Very rare(occurring in less than 1 in 10,000 patients):
- high blood pressure;
- pancreatitis - symptoms include severe abdominal pain, often with nausea (vomiting) or vomiting;
- gastrointestinal bleeding - blood in stool or vomit;
- hallucinations;
- severe vomiting, which can lead to a tear in the part of the digestive tract connecting the mouth to the stomach (esophagus).
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the doctor, pharmacist, or nurse should be informed. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181 C, 02-222 Warsaw
Phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Evertas
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
There are no special storage instructions for the medicine.
The patch (transdermal system) should be stored in the sachet until use.
A damaged or opened patch should not be used.
After removing the patch, it should be folded in half with the adhesive side inwards and pressed firmly. The used patch should be placed in the sachet and then disposed of in a place inaccessible to children. After removing the patch, the patient should not touch their eyes with their fingers until they have washed their hands with soap and water.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Evertas contains
- The active substance of Evertas is rivastigmine. Evertas, 9.5 mg/24 h, transdermal system: each patch releases 9.5 mg of rivastigmine over 24 hours. The patch has a surface area of 9.2 cm and contains 13.8 mg of rivastigmine.
- Other ingredients are:
- 2-ethylhexyl acrylate copolymer and vinyl acetate
- Polyisobutene with medium molecular weight
- Polyisobutene with high molecular weight
- Colloidal anhydrous silica
- Light liquid paraffin
Outer layer:
- Polyethylene/thermoplastic resin/aluminum coated with polyester
Protective layer (removable):
- Polyester coated with fluoropolymer
- Orange ink
What Evertas looks like and what the package contains
Each transdermal system is a thin patch. On the outer, light-brown, covering layer, the text "RIV-TDS 9.5 mg/24 h" is printed in orange ink.
Each patch is in a separate, sealed sachet. Patches are available in packs of 7, 30, 60, or 90 sachets.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Romania, the country of export:
Zentiva, k.s., U kabelovny 130, Dolní Mĕcholupy, 102 37 Prague 10, Czech Republic
Manufacturer:
Luye Pharma AG, Am Windfeld 35, 83714 Miesbach, Germany
Zentiva S.A., B-dul Theodor Pallady nr. 50, Sector 3, Bucharest, Romania
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Romanian marketing authorization number: 11678/2019/01
11678/2019/02
11678/2019/03
11678/2019/04
Parallel import authorization number: 10/20
Date of leaflet approval: 07.11.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Zentiva, k.s.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EvertasDosage form: System, 4.6 mg/24 hActive substance: rivastigmineManufacturer: Luye Pharma AGPrescription requiredDosage form: System, 9.5 mg/24 hActive substance: rivastigmineManufacturer: Luye Pharma AGPrescription requiredDosage form: System, 4.6 mg/24 hActive substance: rivastigminePrescription required
Alternatives to Evertas in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Evertas in Spain
Alternative to Evertas in Ukraine
Online doctors for Evertas
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Evertas – subject to medical assessment and local rules.











