
Evertas
Ask a doctor about a prescription for Evertas

How to use Evertas
Leaflet accompanying the packaging: information for the user
Evertas, 4.6 mg/24 h, transdermal system, patch
Evertas, 9.5 mg/24 h, transdermal system, patch
Evertas, 13.3 mg/24 h, transdermal system, patch
Rivastigmine
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Evertas and what is it used for
- 2. Important information before using Evertas
- 3. How to use Evertas
- 4. Possible side effects
- 5. How to store Evertas
- 6. Contents of the pack and other information
1. What is Evertas and what is it used for
The active substance of Evertas is rivastigmine.
Rivastigmine belongs to a group of substances called cholinesterase inhibitors. In patients
with Alzheimer's-type dementia, there is a decrease in the number of nerve cells
in the brain, which leads to a decrease in the concentration of acetylcholine, a neurotransmitter
(a substance that enables communication between nerve cells), produced by them. The action of rivastigmine
involves blocking the enzymes that cause the breakdown of acetylcholine: acetylcholinesterase and butyrylcholinesterase. By blocking the action of these enzymes, rivastigmine
allows the concentration of acetylcholine in the brain to increase, which helps to alleviate the symptoms of Alzheimer's disease.
Evertas is used to treat adult patients with mild to moderately severe Alzheimer's-type dementia, a progressive brain disease that gradually disrupts memory,
intellectual abilities, and behavior.
2. Important information before using Evertas
When not to use Evertas
- if the patient is allergic to rivastigmine or any of the other ingredients of this medicine (listed in section 6);
- if the patient has an allergic reaction to similar medicines (carbamate derivatives);
- if the patient has a skin reaction that extends beyond the area of skin covered by the patch, if the local reaction worsens (e.g. blisters, exacerbation of skin inflammation, swelling) and if these changes do not resolve within 48 hours after removal of the patch. If such a situation applies to the patient, they should inform their doctor and not apply the Evertas patch.
Warnings and precautions
Before starting to use Evertas, the patient should discuss with their doctor or pharmacist if:
- the patient has or has had heart diseases, such as irregular or slow heart rhythm, prolonged QT interval, family history of prolonged QT interval, torsades de pointes, or low potassium or magnesium levels in the blood;
- the patient has or has had an active stomach ulcer;
- the patient has or has had difficulties in urinating;
- the patient has or has had seizures;
- the patient has or has had asthma or severe respiratory disease;
- the patient has muscle tremors;
- the patient has a low body weight;
- the patient has gastrointestinal reactions, such as nausea (nausea), vomiting, and diarrhea. The patient may become dehydrated (lose too much fluid) if vomiting or diarrhea persists for a longer period;
- the patient has liver function disorders. If any of these situations apply to the patient, closer monitoring by the doctor may be necessary during treatment with this medicine.
If the patient has not applied a patch for more than three days, they should not apply a new patch until they have spoken to their doctor.
Children and adolescents
The use of Evertas in children and adolescents for the treatment of Alzheimer's-type dementia is not appropriate.
Evertas and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Evertas may affect the action of anticholinergic medicines, some of which are used to relieve stomach cramps (e.g. dicyclomine), to treat Parkinson's disease (e.g. amantadine)
or to prevent motion sickness (e.g. diphenhydramine, scopolamine, or meclizine).
Evertas transdermal system, patch should not be used at the same time as metoclopramide (a medicine used to relieve or prevent nausea and vomiting). Taking these two medicines together may cause disorders such as stiffness of the limbs and hand tremors.
If the patient is to undergo surgery during treatment with Evertas transdermal systems, patches, they should tell their doctor about their use, as they may enhance the action of certain muscle relaxants given during anesthesia.
Care should be taken when Evertas transdermal system, patch is used with beta-adrenergic blockers (medicines such as atenolol, used to treat hypertension, angina pectoris, and other heart diseases). Taking these two medicines together may cause disorders such as slowed heart rate (bradycardia) leading to fainting or loss of consciousness.
Care should be taken when Evertas is used with other medicines that may affect heart rhythm or the heart's conduction system (prolonged QT interval).
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
If the patient is pregnant, the benefits of using Evertas should be weighed against the possible effects of the medicine on the unborn child. Evertas should not be used during pregnancy unless absolutely necessary.
During treatment with Evertas transdermal systems, patches, the patient should not breastfeed.
Driving and using machines
The doctor will inform the patient whether their condition allows for safe driving and use of machines. Evertas (patches) may cause fainting or severe confusion. If the patient feels weak or is disoriented, they should not drive vehicles, operate machines, or perform other tasks that require concentration.
3. How to use Evertas
This medicine should always be used exactly as described in the patient leaflet or as directed by the doctor. In case of doubt, the patient should consult their doctor or pharmacist.
IMPORTANT:
- Before applying a new patch, the old patch should be removed.
- Only ONE Evertas patch should be applied per day.
- The patch should not be cut into smaller pieces.
- The patch should be pressed firmly with the palm of the hand for at least
30 seconds.
How to start treatment
The doctor will inform the patient which Evertas patches are best for their case.
- Treatment usually starts with Evertas 4.6 mg/24 h.
- The recommended, usually used daily dose of Evertas is 9.5 mg/24 h. If the treatment is well tolerated, the doctor may consider increasing the dose to 13.3 mg/24 h
- Only one Evertas patch should be applied at a time and changed every 24 hours. During treatment, the doctor may adjust the dose of the medicine to the individual patient's needs.
If the patient has not applied a patch for more than three days, they should not apply a new patch until they have spoken to their doctor. Treatment can be resumed using the same dose if the treatment interruption did not exceed three days. Otherwise, the doctor will recommend resuming treatment with a dose of 4.6 mg/24 h Evertas.
Evertas can be used with food, drink, and alcohol.
Where should the patient apply the Evertas patch
- Before applying the patch, the patient should make sure that the skin in the planned application area is clean, dry, and hairless, free from powder, oils, creams, or liquids that could prevent the patch from sticking properly, free from cuts, rashes, and/or irritations.
- The patient should carefully remove any previously applied patches before applying a new one.Applying multiple patches to the skin may expose the patient to an excessive amount of medicine, which can be dangerous.
- The patient should apply ONEpatch per day to ONLY ONEof the possible locations, as shown in the diagrams:
- upper part of the left arm orupper part of the right arm
- upper part of the chest on the left side oron the right side (avoid applying patches to the breast)
- upper part of the back on the left side oron the right side
- lower part of the back on the left side oron the right side
After 24 hours, the old patch should be removed before applying ONE new patch to
ONLY ONE of the locations shown.
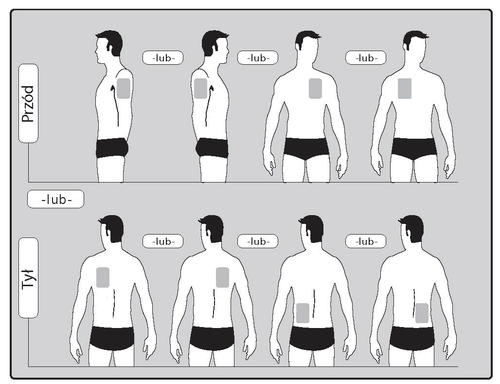
When changing the patch, the patient should remove the previous day's patch before applying a new patch to a different location (e.g. one day on the right side of the body, the next day on the left, one day on the upper part of the body, the next on the lower part). The patient should not apply a patch to the same location before 14 days have passed.
How should the patient apply the Evertas patches
Evertas is a thin, light-brown, plastic patch that is applied to the skin. Each patch is in a tightly closed protective sachet. The patient should not open the sachet or remove the patch until they are ready to apply it to the skin.
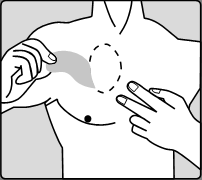
Carefully remove the old patch before applying a new one.
Patient starting treatment for the first time and patients resuming treatment after an interruption of rivastigmine treatment should
start with the actions shown in the second diagram.
Each patch is in a tightly closed protective sachet. The sachet should be opened just before using the patch.
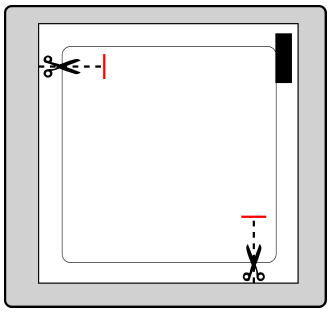
The patient should cut the sachet at both marked places with scissors, but no further than the indicated line. The patient should puncture the sachet to open it. The patient should not cut the sachet along its entire length to avoid damaging the patch.
The patient should remove the patch from the sachet.
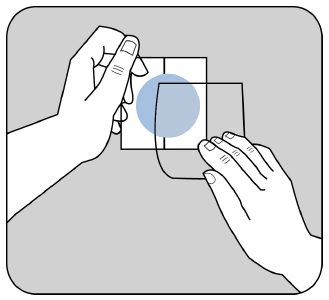
In the case of Evertas 4.6 mg/24 h and 9.5 mg/24 h transdermal system, patch:
Remove the protective layer from the upper, beige-colored side of the patch.
The adhesive side of the patch is protected by a protective layer.
The patient should remove one part of the protective layer without touching the adhesive side of the patch.
In the case of Evertas 13.3 mg/24 h transdermal system, patch:
The adhesive side of the patch is protected by a protective layer.
The patient should remove one part of the protective layer without touching the adhesive side of the patch.
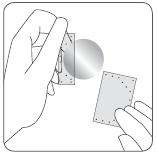
Apply the adhesive side of the patch to the upper or lower part of the back, upper part of the arm, or chest,
and then remove the second part of the protective layer.
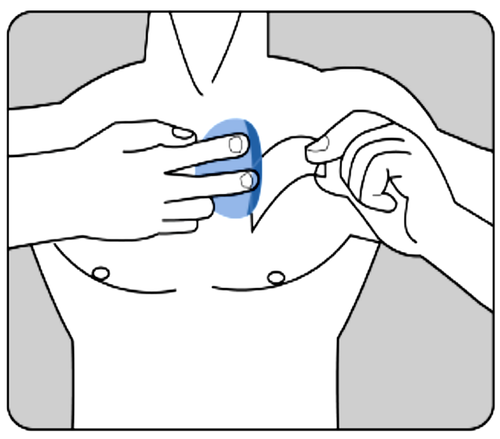
Press the patch firmly with the palm of the hand for at least 30 seconds,
making sure that its edges adhere well to the skin.
The patient can now sign the patch with a pen, e.g. with the name of the day of the week.
The patch should be worn continuously until it is time to change it for a new one. The patient can try different locations
for applying a new patch to find the most convenient and least exposed to abrasion by clothing.
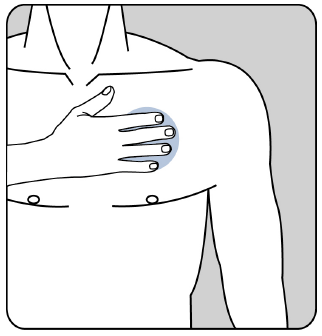
How should the patient remove the Evertas patch
The patient should gently pull one edge of the patch and slowly peel it off the skin. If there are any adhesive residues on the skin, they can be removed by washing the area with warm water and mild soap or baby oil. The patient should not use alcohol or other solvents (nail polish remover and other products).
After removing the patch, the patient should wash their hands with soap and water. If the medicine comes into contact with the eyes or if the eyes become red after contact with the patch, the patient should immediately rinse their eyes with plenty of water, and if the symptoms do not resolve, they should consult a doctor.
Can the patient use Evertas patches while bathing, swimming, or in the sun?
- Bathing, swimming, or showering should not affect the action of the patch. The patient should make sure that the patch does not come loose during these activities.
- The patient should not expose the patch to prolonged external heat sources (e.g. excessive sun exposure, sauna, solarium).
What should the patient do if the patch comes off
If the patch comes off, the patient should apply a new one for the rest of the day and then change it at the usual time the next day.
When and for how long should the patient use Evertas patches
- To get the effect of the treatment, the patient should apply a new patch every day, preferably at the same time every day.
- The patient should apply only one Evertas patch at a time and change it every 24 hours.
What if the patient uses more Evertas than they should
If the patient accidentally applies more than one patch, they should remove all patches,
and then inform their doctor about the accidental application of more than one patch. The patient may need medical attention. In some people who have accidentally taken too much rivastigmine, nausea, vomiting, diarrhea, high blood pressure, and hallucinations have occurred. It may also lead to slowed heart rate and fainting.
What if the patient forgets to use Evertas
If the patient realizes they have forgotten to apply a patch, they should do so as soon as possible. The next patch can be applied at the usual time the next day. The patient should not apply two patches to make up for a missed dose.
What if the patient stops using Evertas
If the patient stops using the medicine, they should inform their doctor or pharmacist.
If the patient has any further questions about the use of this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects occur more frequently during the initial period of use of the medicine or during the period of dose increase. Side effects usually subside slowly as the body adapts to the treatment.
If the patient experiences any of the following side effects, they should remove the patch and immediately tell their doctor, as these effects can be serious:
Common(occurring in less than 1 in 10 patients):
- loss of appetite;
- dizziness;
- agitation or drowsiness;
- urinary incontinence (inability to properly retain urine).
Uncommon(occurring in less than 1 in 100 patients):
- heart rhythm disorders, such as slow heart rate;
- seeing things that are not there (hallucinations);
- stomach ulcers;
- dehydration (loss of too much fluid);
- excessive restlessness (high level of activity, agitation);
- aggression.
Rare(occurring in less than 1 in 1,000 patients):
- falls.
Very rare(occurring in less than 1 in 10,000 patients):
- stiffness of the arms or legs;
- hand tremors.
Frequency not known(cannot be estimated from the available data):
- allergic reaction at the patch application site, such as blisters or skin inflammation;
- worsening of Parkinson's disease symptoms - such as tremors, stiffness, or dragging of the legs;
- pancreatitis - symptoms include severe pain in the upper abdomen, often with nausea (nausea) or vomiting;
- rapid or irregular heartbeat;
- high blood pressure;
- seizures (convulsions);
- liver function disorders (jaundice, yellowing of the whites of the eyes, unexplained dark urine or nausea, vomiting, fatigue, and loss of appetite);
- changes in liver function test results;
- anxiety,
- nightmares,
- Pisa syndrome (a condition involving involuntary muscle contraction with abnormal tilting of the body and head to one side).
If the patient experiences any of the above side effects, they should remove the patch and immediately tell their doctor.
Other side effects reported after using rivastigmine in capsule or oral solution form, which may also occur after using patches:
Common(occurring in less than 1 in 10 patients):
- excessive salivation;
- loss of appetite;
- anxiety;
- general malaise;
- tremors or confusion;
- excessive sweating.
Uncommon(occurring in less than 1 in 100 patients):
- heart rhythm disorders (e.g. rapid heartbeat);
- difficulty falling asleep;
- accidental falls.
Rare(occurring in less than 1 in 1,000 patients):
- seizures (convulsions);
- ulcerative disease of the intestines;
- chest pain - may be caused by a heart spasm.
Very rare(occurring in less than 1 in 10,000 patients):
- high blood pressure;
- pancreatitis - symptoms include severe pain in the upper abdomen, often with nausea (nausea) or vomiting;
- gastrointestinal bleeding - blood in stool or vomit;
- seeing things that are not there (hallucinations);
- severe vomiting, which can lead to rupture of the part of the digestive tract connecting the mouth to the stomach (esophagus).
Reporting side effects
If side effects occur, including any not listed in this leaflet, the patient should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw.
Phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative in Poland.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Evertas
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and sachet after “Expiry Date (EXP)”. The expiry date refers to the last day of the month.
There are no special precautions for storing the medicine.
Before use, the patch should be stored in the sachet.
Do not use a patch that is damaged or shows signs of opening.
After removing the patch, the patient should fold it in half with the adhesive side inwards and press firmly. The used patch should be placed in the sachet and then discarded in a place inaccessible to children. After removing the patch, the patient should not touch their eyes with their fingers before washing their hands with soap and water.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Evertas contains
- The active substance of Evertas is rivastigmine. Evertas, 4.6 mg/24 h, transdermal system: each patch releases 4.6 mg of rivastigmine over 24 hours. The patch has an area of 4.6 cm and contains 6.9 mg of rivastigmine. Evertas, 9.5 mg/24 h, transdermal system: each patch releases 9.5 mg of rivastigmine over 24 hours. The patch has an area of 9.2 cm and contains 13.8 mg of rivastigmine. Evertas, 13.3 mg/24 h, transdermal system: each patch releases 13.3 mg of rivastigmine over 24 hours. The patch has an area of 12.8 cm and contains 19.2 mg of rivastigmine.
- Other ingredients are:
Active layer:
- 2-ethylhexyl acrylate copolymer and vinyl acetate
Adhesive matrix layer:
- polyisobutene with medium molecular weight
- polyisobutene with high molecular weight
- anhydrous colloidal silica
- light liquid paraffin
Outer layer:
- polyethylene/thermoplastic resin/aluminum coated with polyester
Protective layer (removable):
- polyester coated with fluoropolymer
- orange ink
What Evertas looks like and contents of the pack
Each transdermal system is a thin, round, light-brown plastic patch. On the outer, light-brown covering layer, there is an orange inscription:
- “RIV-TDS 4.6 mg/24 h”
- “RIV-TDS 9.5 mg/24 h”
- “RIV-TDS 13.3 mg/24 h”
Each patch is in a separate, tightly closed protective sachet.
Evertas, 4.6 mg/24 h, transdermal system
Evertas, 9.5 mg/24 h, transdermal system
Patches are available in packs of 7 or 30 transdermal systems, as well as in bulk packs of 60 (2x30) or 90 (3x30) transdermal systems.
Evertas, 13.3 mg/24 h, transdermal system
Patches are available in packs of 30 transdermal systems, as well as in bulk packs of 60 (2x30) or 90 (3x30) transdermal systems.
Not all pack sizes may be marketed.
Marketing authorization holder
Zentiva, k.s., U kabelovny 130, Dolní Mĕcholupy, 102 37 Prague 10, Czech Republic
Manufacturer
Luye Pharma AG, Am Windfeld 35, 83714 Miesbach, Germany
S.C. Zentiva S.A, B-dul Theodor Pallady nr.50, sector 3, 032266 Bucharest, Romania
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Poland
Evertas
Romania Evertas 4.6 mg/24 h transdermal patch
Romania Evertas 9.5 mg/24 h transdermal patch
For further information about this medicine, the patient should contact the representative of the marketing authorization holder in Poland:
Zentiva Poland Sp. z o.o.
Bonifraterska 17 street
00-203 Warsaw
phone: +48 22 375 92 00
Date of last revision of the leaflet:February 2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterLuye Pharma AG Zentiva SA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EvertasDosage form: System, 4.6 mg/24 hActive substance: rivastigmineManufacturer: Luye Pharma AGPrescription requiredDosage form: System, 9.5 mg/24 hActive substance: rivastigmineManufacturer: Luye Pharma AGPrescription requiredDosage form: System, 4.6 mg/24 hActive substance: rivastigminePrescription required
Alternatives to Evertas in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Evertas in Spain
Alternative to Evertas in Ukraine
Online doctors for Evertas
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Evertas – subject to medical assessment and local rules.











