
How to use Divina
Package Leaflet: Information for the Patient
Read the package leaflet carefully before using the medicine, as it contains
important information for the patient.
- The package leaflet should be kept in case it needs to be read again.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed strictly for a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Divina and what is it used for
- 2. Important information before using Divina
- 3. How to use Divina
- 4. Possible side effects
- 5. How to store Divina
- 6. Contents of the pack and other information
1. What is Divina and what is it used for
Divina is a cyclic, two-phase medicine intended for use in hormone replacement therapy (HRT). The medicine contains two types of female hormones, estradiol valerate (estrogen) and medroxyprogesterone acetate (progestogen).
Divina is used to:
Relieve symptoms occurring after menopause
During menopause, the amount of estrogen produced by the woman's body decreases. This can lead to symptoms such as a feeling of heat on the face, neck, and chest ("hot flashes"). Divina relieves these symptoms that occur after menopause. Divina is prescribed only in cases where the symptoms significantly negatively affect the patient's daily life.
Prevention of osteoporosis
After menopause, some women develop brittle bones (osteoporosis). All available options should be discussed with the doctor. If the patient is at increased risk of fractures due to osteoporosis and other medicines are not suitable, Divina can be used to prevent osteoporosis.
Experience in treating patients over 65 years of age is limited.
2. Important information before using Divina
Medical history and regular check-ups
The use of hormone replacement therapy (HRT) is associated with a risk that should be considered when deciding to start or continue therapy.
Cases of using therapy in women with premature menopause (caused by ovarian failure or surgical procedures) are rare. In the case of women with premature menopause, the risk of using HRT may be different. A doctor should be consulted.
Before starting (or reusing) HRT, the doctor will conduct a medical interview about the patient and her family. The doctor may also perform a physical examination, including a breast examination and (or) internal organs, if necessary.
After starting treatment with Divina, regular check-ups should be attended (at least once a year). During these visits, the benefits and risks of continued use of Divina should be discussed with the doctor.
Regular breast examinations should be performed in accordance with the doctor's recommendations.
When not to use Divina:
If any of the following points apply to the patient or if the patient has doubts about any of the following points, they should consult a doctorbefore using Divina.
Divina should not be used:
- if the patient is allergic to active substancesor any of the other ingredients of this medicine (listed in section 6),
- if the patient has or has had malignant breast canceror if there is a suspicion of it,
- if the patient has estrogen-sensitive malignant cancer, such as endometrial cancer (endometrium) or if there is a suspicion of it,
- if there is vaginal bleeding of unknown cause,
- if the patient has untreated endometrial hyperplasia(thickening of the uterine lining),
- if the patient has or has had venous thrombosis(venous thromboembolic disease) of the lower limbs (deep vein thrombosis) or lungs (pulmonary embolism),
- if the patient has a blood clotting disorder(such as protein C, protein S, or antithrombin deficiency),
- if the patient has or has recently had arterial thromboembolic disorders, such as heart attack or stroke,
- if the patient has or has had liver diseaseand liver function tests have not returned to normal,
- if the patient has a rare inherited blood disorder called porphyria.
If any of the above factors occur for the first time while using Divina, the medicine should be stopped immediately and the doctor should be contacted without delay.
Warnings and precautions
Before starting Divina, the doctor or pharmacist should be consulted.
Before starting treatment, the doctor should be informed about any current or past conditions, as they may recur or worsen during treatment with Divina. In the following cases, the frequency of check-ups should be increased:
- uterine fibroids (uterine fibroids),
- endometriosis or previous cases of endometrial hyperplasia,
- increased risk of thromboembolic disorders (see "Blood clots in the veins (thrombosis)"),
- increased risk of estrogen-sensitive cancer (e.g., if the patient's mother, sister, or grandmother had breast cancer),
- high blood pressure,
- liver function disorders, such as a benign liver tumor,
- diabetes with vascular complications or without,
- gallstones,
- severe headaches or migraines,
- systemic lupus erythematosus (SLE), a disease that affects many organs in the body,
- epilepsy,
- asthma,
- otosclerosis, a disease that affects the eardrum and hearing,
- very high levels of triglycerides in the blood,
- fluid retention due to heart or kidney problems,
- inherited or acquired angioedema (a condition characterized by swelling, usually of the face, limbs, and joints).
Stop using Divina and contact a doctor immediately
If the patient experiences any of the following symptoms while using HRT:
- any of the diseases listed in the "When not to use Divina" section,
- yellowing of the skin or whites of the eyes (jaundice); these may be signs of liver disease,
- swelling of the face, tongue, and (or) throat, and (or) difficulty swallowing or hives, along with difficulty breathing, which may indicate angioedema,
- significant increase in blood pressure (possible symptoms include headaches, fatigue, dizziness),
- migraines that occur for the first time,
- if the patient becomes pregnant,
- if signs of a blood clot appear, such as:
- painful swelling and redness of the lower limbs,
- sudden chest pain,
- difficulty breathing.
Note:Divina is not a contraceptive. If it has been less than 12 months since the last menstrual period or if the patient is under 50 years old, additional contraceptive measures may be necessary to prevent pregnancy. A doctor should be consulted.
HRT and cancer
Endometrial hyperplasia (thickening of the uterine lining) and endometrial cancer (cancer of the uterine lining)
The use of estrogen-only HRT increases the risk of endometrial hyperplasia and endometrial cancer.
The use of estrogens in combination with progestogen for at least 12 days per cycle or continuous combined therapy reduces this additional risk.
In women with an intact uterus who do not use HRT, endometrial cancer is diagnosed in approximately 5 out of 1000 women between the ages of 50 and 65.
In women with an intact uterus who use estrogen-only HRT, endometrial cancer is diagnosed in 10 to 60 out of 1000 women (i.e., 5 to 55 additional cases), depending on the dose and duration of treatment.
Irregular bleeding
Irregular bleeding or spotting may occur during the first 3-6 months of using Divina. If irregular bleeding:
- lasts longer than the first 6 months,
- occurs when the patient has been using Divina for more than 6 months,
- persists after stopping Divina, the doctor should be consulted immediately.
Breast cancer
Data confirm that taking hormone replacement therapy (HRT) in the form of a combination of estrogen and progestogen or estrogen alone increases the risk of breast cancer. The additional risk depends on how long the patient uses HRT. This additional risk becomes apparent after 3 years of HRT. After stopping HRT, the additional risk will decrease over time, but the risk may persist for 10 years or longer if HRT lasted more than 5 years.
Comparison
In women between the ages of 50 and 54 who do not use HRT, breast cancer will be diagnosed in approximately 13 to 17 out of 1000 women over a 5-year period.
In women aged 50 who start 5-year estrogen-only HRT, the number of cases will be 16-17 out of 1000 patients (i.e., 0 to 3 additional cases).
In women aged 50 who start 5-year combined estrogen-progestogen HRT, the number of cases will be 21 out of 1000 patients (i.e., 4 to 8 additional cases).
In women between the ages of 50 and 59 who do not use HRT, breast cancer will be diagnosed in approximately 27 out of 1000 women over a 10-year period.
In women aged 50 who start 10-year estrogen-only HRT, the number of cases will be 34 out of 1000 patients (i.e., 7 additional cases).
In women aged 50 who start 10-year combined estrogen-progestogen HRT, the number of cases will be 48 out of 1000 patients (i.e., 21 additional cases).
Regular breast examinations should be performed.The doctor should be consulted if changes such as:
- wrinkles on the skin,
- changes in the breast area,
- any visible or palpable thickening.
In addition, participation in offered breast cancer screening programs is recommended.
It is essential to inform the medical staff performing the mammography about the use of HRT, as this medicine may cause an increase in the radiological density of the breast, which may affect the mammography result. In areas with increased density, mammography may not detect all tumors.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of HRT containing only estrogens or a combination of estrogens and progestogens is associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer depends on age. For example, in women between the ages of 50 and 54 who do not use HRT, ovarian cancer will be diagnosed over a 5-year period in approximately 2 out of 2000 women. In women who have taken HRT for 5 years, it will occur in approximately 3 out of 2000 patients (i.e., 1 additional case).
Effect of HRT on the heart and circulation
Blood clots in the veins (thrombosis)
The risk of developing venous thromboembolic diseaseis approximately 1.3-3 times higher in women using HRT than in those who do not use it, especially in the first year of therapy.
Blood clots can have serious consequences and may cause chest pain, shortness of breath, fainting, or even death if they enter the lungs.
The likelihood of blood clots in the veins increases with age and depends on the presence of the following factors. The doctor should be informed if any of the following situations occur:
- the patient is unable to walk for an extended period due to a serious surgical procedure, injury, or illness (see also section 3 "If you need surgery"),
- the patient is obese (BMI >30 kg/m),
- the patient has a blood clotting disorder that requires long-term treatment with anticoagulant medication,
- any close relatives have had a blood clot in the lower limb, lung, or other organ,
- the patient has had extensive surgery,
- the patient has systemic lupus erythematosus (SLE),
- the patient has cancer,
- if the patient is pregnant or postpartum.
Symptoms of a blood clot can be found in the "Stop using Divina and contact a doctor immediately" section.
Comparison
In women between the ages of 50 and 59 who do not use HRT, a blood clot in the veins will occur in approximately 4 to 7 out of 1000 women over a 5-year period.
In women between the ages of 50 and 59 who have used combined estrogen-progestogen HRT for more than 5 years, a blood clot in the veins will occur in 9 to 12 out of 1000 patients (i.e., 5 additional cases).
Heart disease (heart attack)
There is no scientific evidence that HRT can prevent heart attacks.
Women over 60 years old who use combined estrogen-progestogen HRT are slightly more likely to develop heart disease than those who do not use HRT.
Stroke
The risk of stroke is approximately 1.5 times higher in women using HRT than in those who do not use it. The number of additional stroke cases due to HRT increases with age.
Comparison
In women between the ages of 50 and 59 who do not use HRT, a stroke will occur in approximately 8 out of 1000 women over a 5-year period. In women between the ages of 50 and 59 who use HRT, a stroke will occur in 11 out of 1000 patients over a 5-year period (i.e., 3 additional cases).
Other conditions
- HRT does not prevent cognitive decline. There is evidence of an increased risk of dementia in women who start HRT at an age over 65. A doctor should be consulted.
- Estrogens may cause fluid retention, especially in patients with heart or kidney problems.
- HRT may affect the results of certain endocrine tests and liver function tests. The doctor should be informed about the use of Divina before performing these tests.
- The patient should inform the doctor if they know they have high triglyceride levels in the blood. In very rare cases, significant increases in triglyceride levels in the blood during estrogen therapy may lead to pancreatitis.
- Divina does not prevent pregnancy. Therefore, it should not be used as a contraceptive.
Divina and other medicines
Some medicines may interfere with the action of Divina. This may lead to irregular bleeding. This applies to the following medicines:
- medicines used to treat epilepsy(such as phenobarbital, phenytoin, and carbamazepine),
- medicines used to treat tuberculosis(such as rifampicin, rifabutin),
- medicines used to treat HIV or hepatitis C
- (such as protease inhibitors and non-nucleoside reverse transcriptase inhibitors (e.g., nevirapine, efavirenz, ritonavir, and nelfinavir)),
- herbal medicines containing St. John's wort ( Hypericum perforatum).
Hormone replacement therapy with estrogen may affect how other medicines work. This applies to the following medicines:
- medicines used to treat epilepsy- lamotrigine, as it may increase the risk of seizures.
- medicines used to treat hepatitis C(such as the combination therapy of ombitasvir/paritaprevir/ritonavir with dasabuvir or without dasabuvir and the combination therapy of glecaprevir/pibrentasvir), as they may cause an increase in liver function test results [increase in alanine aminotransferase (ALT) enzyme activity] in women using combined hormonal contraceptives containing ethinyl estradiol. It is not known whether the concurrent use of Divina with these hepatitis C treatment regimens may increase ALT enzyme activity.
The doctor or pharmacist should be informed about all medicines currently being taken, recently taken, or potentially taken in the future, including those available without a prescription, herbal medicines, or other natural products. The doctor will provide advice on this matter.
Laboratory tests
If a blood test is necessary, the laboratory staff should be informed about the use of Divina, as it may affect the results of some tests.
Pregnancy and breastfeeding
Divina is intended for use only in postmenopausal women. If pregnancy occurs, the use of Divina should be stopped and a doctor should be consulted.
The use of Divina is contraindicated during breastfeeding.
Driving and using machines
No effect of the medicine on the ability to drive and use machines has been observed.
Divina contains lactose.
If the patient has previously been diagnosed with intolerance to some sugars, they should consult a doctor before taking the medicine.
One white tablet contains 86.6 mg of lactose, and one blue tablet contains 72.0 mg of lactose.
3. How to use Divina
This medicine should always be used according to the doctor's instructions. In case of doubts, the doctor should be consulted. The doctor will try to prescribe the smallest dose necessary for the treatment of symptoms, for the shortest possible period. If the patient thinks the dose is too high or too low, they should inform the doctor.
Divina should be taken according to the information on the calendar pack, one tablet per day in 21-day cycles without interruption, followed by a 7-day break. During this break, most women experience withdrawal bleeding similar to menstruation. It is recommended to take the tablet in the evening.
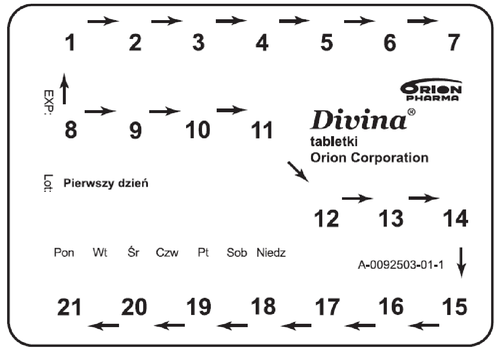
- 1. Each blister pack has the inscription "First day" along with the marked days of the week. The day of the week when treatment starts should be marked on the blister pack (e.g., by making a hole). This allows for easy verification that the tablets have been taken according to the schedule.
- 2. Postmenopausal women can start treatment immediately. Women who are still menstruating should start treatment on the fifth day of their menstrual cycle (on the fifth day from the start of menstruation).
- 3. In women who are not using HRT or in women switching from another combined HRT product to continuous HRT, treatment with Divina can be started on any convenient day.
- 4. In women switching from cyclic or continuous sequential HRT, treatment should be started the day after the end of the previous treatment cycle (lasting 28 days).
- 5. The tablets should be taken in the order shown on the packaging - first the white tablets, then the blue tablets. After this period, there is a 7-day break. During this time, most women experience withdrawal bleeding.
- 6. The next cycle of tablet intake should be started on the same day of the week as the first cycle. It is possible that some women will not experience bleeding during the 7-day break in therapy or will experience bleeding only in some cycles.
In case of bleeding
During the first few months of treatment, bleeding or spotting from the genital tract may occur. The doctor should be consulted if prolonged or irregular bleeding occurs. It may be helpful to keep a record of the dates of bleeding, as the doctor may ask about their occurrence during check-ups.
If the patient feels that the effect of Divina is too strong or insufficient, they should not change the dose or stop taking the medicine without consulting a doctor first.
If surgery is required
Before surgery, the surgeon should be informed about the use of Divina. It may be necessary to stop using Divina 4 to 6 weeks before surgery to reduce the risk of blood clots (see section 2 "Blood clots in the veins"). A doctor should be consulted regarding when to restart Divina.
Overdose of Divina
No serious symptoms of overdose have been observed after taking high doses of oral HRT medications. Estrogen overdose may cause nausea, headaches, and bleeding from the genital tract. If a higher dose than recommended is taken, a doctor or pharmacist should be consulted immediately.
Missing a dose of Divina
If a tablet is not taken, it should be taken the next morning, and the tablet scheduled for that day should be taken in the evening. If a tablet is missed altogether, treatment should be continued with the next tablet. There may be minor bleeding during the cycle.
When planning a longer trip, the patient should ensure they have a sufficient supply of tablets.
A double dose should not be taken to make up for a missed dose.
Stopping Divina
In case of any further doubts about the use of this medicine, a doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, Divina can cause side effects, although not everybody gets them.
Divina may cause side effects, especially during the first few months of treatment. These are usually mild and disappear during treatment.
The most common side effects of Divina are headache and breast tenderness (in 10% of patients).
The following diseases are reported more frequently in women using HRT than in those who do not use it:
- breast cancer,
- abnormal growth or cancer of the uterine lining (endometrial hyperplasia or endometrial cancer),
- ovarian cancer,
- blood clots in the veins of the lower limbs or lungs (venous thromboembolic disease),
- heart disease,
- stroke,
- increased risk of dementia if HRT is started at an age over 65.
More information about the above side effects can be found in section 2.
Common (may affect up to 1 in 10 people)
- weight gain,
- weight loss,
- headaches, dizziness,
- nausea, vomiting, stomach cramps, gas,
- discharge, vulvovaginitis, menstrual disorders,
- skin irritation, itching at the injection site, pain, increased sweating,
- breast tenderness or pain,
- bleeding or spotting, irregular periods,
- depression, nervousness, lethargy,
- edema,
- hot flashes.
Uncommon (may affect up to 1 in 100 people)
- mood changes, changes in libido, anxiety, insomnia, apathy, emotional instability, concentration disorders, euphoria, agitation,
- migraine, numbness, seizures,
- vision disorders, dry eyes,
- hypertension, superficial thrombophlebitis, purpura,
- shortness of breath, runny nose,
- benign breast tumor or endometrial tumor,
- increased appetite, high cholesterol levels in the blood,
- palpitations,
- constipation, indigestion, diarrhea, anal disorders,
- acne, hair loss, dry skin, nail disorders, skin nodules, excessive hair growth, erythema nodosum, hives,
- joint disorders, muscle cramps,
- increased urination and (or) increased need to urinate, urinary incontinence, cystitis, urine discoloration, hematuria,
- breast tenderness or swelling, endometrial hyperplasia, uterine disease,
- fatigue, abnormal laboratory test results, weakness, fever, flu-like symptoms, malaise,
- hypersensitivity.
Rare (may affect up to 1 in 1000 people)
- venous thromboembolic disease (e.g., deep vein thrombosis of the lower limbs or pulmonary embolism),
- liver function disorders and bile flow disorders,
- rash,
- intolerance to contact lenses,
- painful menstruation, premenstrual syndrome.
Unknown (frequency cannot be estimated from available data)
- uterine fibroids,
- exacerbation of inherited or acquired angioedema,
- cerebrovascular disorders (episodes of cerebral ischemia),
- abdominal pain, bloating,
- liver disease causing yellowing of the skin (cholestatic jaundice),
- urticaria.
The following side effects have been observed in other HRTs:
- heart attack,
- gallbladder disease,
- various skin and subcutaneous tissue disorders:
- skin discoloration, especially on the face or neck, known as "pregnancy spots" (chloasma),
- painful skin nodules (erythema nodosum),
- rash with ring-shaped or ulcerative lesions (erythema multiforme).
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, the doctor or pharmacist should be informed.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Divina
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiration date stated on the carton after "Expiration Date (EXP)". The expiration date refers to the last day of the specified month.
The medicine should not be stored at temperatures above 25°C.
Medicines should not be disposed of via wastewater or household waste. A pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Divina contains
One white tablet contains:
- The active substance is: 2 mg estradiol valerate.
- Other ingredients are: lactose monohydrate, cornstarch, gelatin, talc, magnesium stearate.
One blue tablet contains:
- The active substances are: 2 mg estradiol valerate and 10 mg medroxyprogesterone acetate.
- Other ingredients are: lactose monohydrate, cornstarch, indigo carmine (E 132), gelatin, povidone, talc, magnesium stearate.
What Divina looks like and contents of the pack
Package size
The blister pack contains 21 tablets (11 white + 10 blue).
1 or 3 blister packs in a cardboard box.
Marketing authorization holder and manufacturer
Marketing authorization holder
CS 50070
Lys Lez Lannoy 59452
France
For more information about this medicine, please contact the local representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o.o.
[email protected]
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterDELPHARM LILLE S.A.S. Orion Corporation
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DivinaDosage form: Tablets, 2 mg (white), 2 mg + 0.5 mg (light brown)Active substance: norgestrel and estrogenManufacturer: Bayer AG Bayer Weimar GmbH und Co. KGPrescription requiredDosage form: Tablets, 1 mg (white), 1 mg + 10 mg (grey)Active substance: dydrogesterone and estrogenManufacturer: Abbott Biologicals B.V.Prescription requiredDosage form: Tablets, 1 mg (white), 1 mg + 10 mg (grey)Active substance: dydrogesterone and estrogenPrescription required
Alternatives to Divina in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Divina in Spain
Online doctors for Divina
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Divina – subject to medical assessment and local rules.






