

Dezaftan med

Ask a doctor about a prescription for Dezaftan med

How to use Dezaftan med
Leaflet attached to the packaging: patient information
Dezaftan med
(2.9 mg + 1.96 mg + 25.6 mg)/ml, spray for oral use, solution
Cetylpyridinium chloride + Lidocaine hydrochloride monohydrate + Zinc gluconate
It is necessary to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any not listed in this leaflet, the doctor or pharmacist should be informed. See section 4.
- If there is no improvement after 5 days or the patient feels worse, a doctor should be consulted.
Table of contents of the leaflet
- 1. What is Dezaftan med and what is it used for
- 2. Important information before using Dezaftan med
- 3. How to use Dezaftan med
- 4. Possible side effects
- 5. How to store Dezaftan med
- 6. Contents of the packaging and other information
1. What is Dezaftan med and what is it used for
Dezaftan med is a spray for oral use. It has antiseptic (disinfecting) and local anesthetic effects.
It contains three active substances:
- cetylpyridinium chloride, which has disinfecting, antibacterial, antifungal, and antiviral effects;
- lidocaine hydrochloride monohydrate, which has a local anesthetic effect, relieving pain;
- zinc gluconate, which supports the body's immune system.
Indications for use
An antiseptic and local anesthetic product with zinc for use:
- in inflammatory conditions of the oral cavity;
- in gingivitis;
- in infections and ulcers of the oral mucosa (aphthae, thrush), including those resulting from injuries caused by orthodontic appliances and dental prostheses.
If there is no improvement after 5 days or the patient feels worse, a doctor should be consulted.
2. Important information before using Dezaftan med
When not to use Dezaftan med
- if the patient is allergic to cetylpyridinium chloride, lidocaine hydrochloride monohydrate, zinc gluconate (active substances of the medicine), other local anesthetics from the amide group, or any of the other ingredients of this medicine (listed in section 6);
- if the patient has severe liver or kidney failure;
- if the patient has methemoglobinemia - a very rare condition in which the hemoglobin in the blood loses its ability to carry oxygen to the body's cells;
- in children under 6 years of age;
- if the patient has myasthenia gravis (a disease characterized by excessive fatigue and muscle weakness).
Warnings and precautions
Before starting to use Dezaftan med, the patient should discuss it with their doctor if they have:
- pharyngitis and fever lasting several days before using Dezaftan med;
- sore throat with fever, dizziness, nausea, or vomiting.
Dezaftan med should not be used for more than 5 consecutive days. If no improvement is observed after 5 days of use, the patient should consult a doctor immediately, as prolonged or recurrent aphthae may be the first symptom of a serious disease.
Lidocaine hydrochloride monohydrate, one of the ingredients of Dezaftan med, may cause swallowing disorders and increase the risk of choking (see also the section "Using Dezaftan med with food and drink").
Children
Dezaftan med should not be used in children under 6 years of age.
Dezaftan med and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
In particular, the patient should inform their doctor if they are taking:
- acetylsalicylic acid (a component of medicines used to lower fever);
- sulfonamides (medicines used to treat bacterial infections);
- cimetidine (a medicine used to treat stomach and duodenal ulcers);
- beta-blockers (medicines used to treat high blood pressure and coronary heart disease, e.g., propranolol);
- norepinephrine (a medicine used in resuscitation and treatment of anaphylactic shock);
- inhalation anesthetics (medicines used for general anesthesia);
- barbiturates (medicines used to treat insomnia and epilepsy);
- rifampicin (an antibiotic used to treat tuberculosis and some other infections);
- phenytoin (a medicine used to treat epilepsy);
- muscle relaxants (e.g., pancuronium);
- tetracycline antibiotics (medicines used to treat bacterial infections);
- chelating agents (medicines used to treat metal poisoning, e.g., D-penicillamine);
- ibuprofen or indomethacin (painkillers belonging to the group of nonsteroidal anti-inflammatory drugs - NSAIDs);
- thiazide diuretics (medicines that increase urine production);
- corticosteroids (medicines used to treat rheumatic diseases);
- medicines and dietary supplements containing calcium, iron, and copper.
Using Dezaftan med with food and drink
Dezaftan med should not be used while eating or immediately before eating, due to the risk of choking. The medicine is best used 1 hour before or 1 hour after a meal.
Care should be taken when drinking hot beverages and eating hot foods.
Due to reduced sensitivity to heat, there is an increased risk of burning the oral mucosa and throat.
During the use of Dezaftan med, dairy products should not be consumed, as they reduce zinc absorption.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
Dezaftan med is not recommended for use during pregnancy or in women of childbearing age who do not use effective contraception.
The medicine should not be used in breastfeeding women.
Driving and using machines
There is no data on the effect of the medicine on the ability to drive and use machines.
Dezaftan med contains ethanol
This medicine contains 27 mg of alcohol (ethanol 96%) in 3 doses of the medicine (0.51 ml), which is equivalent to 27 mg/0.51 ml (5.1% [m/v]). The amount of alcohol in 3 doses (0.51 ml) of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine.
The small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Dezaftan med
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor or pharmacist. If in doubt, the patient should consult a doctor or pharmacist.
Dezaftan med is used locally, spraying the surface of the oral mucosa.
Duration of use
The medicine should not be used for more than 5 consecutive days without consulting a doctor.
Recommended dose
Adults:
use 3 sprays every 1 or 2 hours.
Do not use more than 8 times a day.
Children over 6 years of age:
use 3 sprays every 2 or 3 hours.
Do not use more than 6 times a day.
Instructions for administering Dezaftan med
Before the first use of the medicine, the pump should be pressed 6 times energetically, holding the container vertically, with the nozzle pointing away from the patient, to fill the dispenser and obtain the correct spray.
If the medicine has not been used for 2 days or longer, the pump should be prepared again by spraying the aerosol 3 times, holding the container vertically, with the nozzle pointing away from the patient.
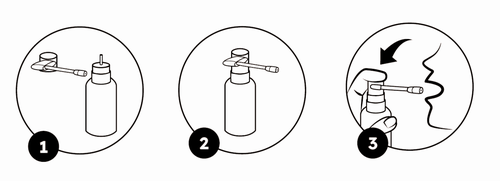
The medicine requires the attachment of a dispenser tip before the first use – fig. 1.
- 1. Attach the dispenser tip as shown in fig. 2.
- 2. Direct the dispenser tip to the oral cavity – as shown in fig. 3.
- 3. Press the dispenser firmly with the thumb or index finger. Repeat this action the appropriate number of times, according to the recommended dosage.
Use in children
Dezaftan med should not be used in children under 6 years of age.
Overdose of Dezaftan med
In case of overdose, the patient should consult a doctor or pharmacist.
The following symptoms of overdose may occur:
nausea, vomiting, abdominal pain, diarrhea, metallic taste in the mouth, headache, collapse (circulatory arrest with loss of consciousness), convulsions, coma.
Missed dose of Dezaftan med
A double dose should not be used to make up for a missed dose.
If the patient has any further doubts about the use of this medicine, they should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Dezaftan med can cause side effects, although not everybody gets them.
The following side effects may occur
Uncommon(may affect up to 1 in 100 people):
- local skin reactions, such as: redness (local skin redness), itching, rash, burning.
Rare(may affect up to 1 in 1,000 people):
- severe skin reactions, such as: blistering or pustular rash, which may spread or become generalized;
- urticaria (light red, itchy blisters on the skin);
- increased sensitivity to sunlight;
- headache;
- gastrointestinal disorders (nausea, abdominal pain, diarrhea);
- vomiting, metallic taste in the mouth;
- inflammation of the oral mucosa.
Very rare(may affect up to 1 in 10,000 people):
- worsening of existing kidney failure.
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should inform their doctor or pharmacist.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Dezaftan med
The medicine should be stored out of sight and reach of children.
There are no special precautions for storing the medicine.
The medicine should not be used after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
The shelf life after first opening is 3 months.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Dezaftan med contains
- The active substances of the medicine are: cetylpyridinium chloride, lidocaine hydrochloride monohydrate, zinc gluconate. 1 ml of the spray contains: 2.9 mg of cetylpyridinium chloride, 1.96 mg of lidocaine hydrochloride monohydrate, and 25.6 mg of zinc gluconate (which corresponds to 3.7 mg of zinc ions). A single sprayed dose contains 0.17 ml of the solution. 3 doses (0.51 ml of the solution) contain 1.5 mg of cetylpyridinium chloride, 1.0 mg of lidocaine hydrochloride monohydrate, and 13.065 mg of zinc gluconate (which corresponds to 1.875 mg of zinc ions).
- The other ingredients are: levomenthol, ethanol 96%, glycerol, sucralose (E 955), purified water.
What Dezaftan med looks like and what the pack contains
Dezaftan med is a colorless solution with a minty taste and smell.
The packaging of the medicine is a HDPE polyethylene bottle with a spray pump (LDPE polyethylene, PP polypropylene, silicone, stainless steel) and a throat applicator (PP polypropylene) with a dose of 0.17 ml, in a cardboard box.
1 bottle of 30 ml
Marketing authorization holder and manufacturer
Aflofarm Farmacja Polska Sp. z o.o.
Partyzancka 133/151
95-200 Pabianice
Phone: +48 42 22-53-100
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAflofarm Farmacja Polska Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Dezaftan med
Alternatives to Dezaftan med in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Dezaftan med in Ukraine
Alternative to Dezaftan med in Spain
Online doctors for Dezaftan med
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Dezaftan med – subject to medical assessment and local rules.














