
Citotec

Ask a doctor about a prescription for Citotec

How to use Citotec
PATIENT INFORMATION LEAFLET: USER INFORMATION
Warning! Keep the leaflet, the information on the immediate packaging is in a foreign language.
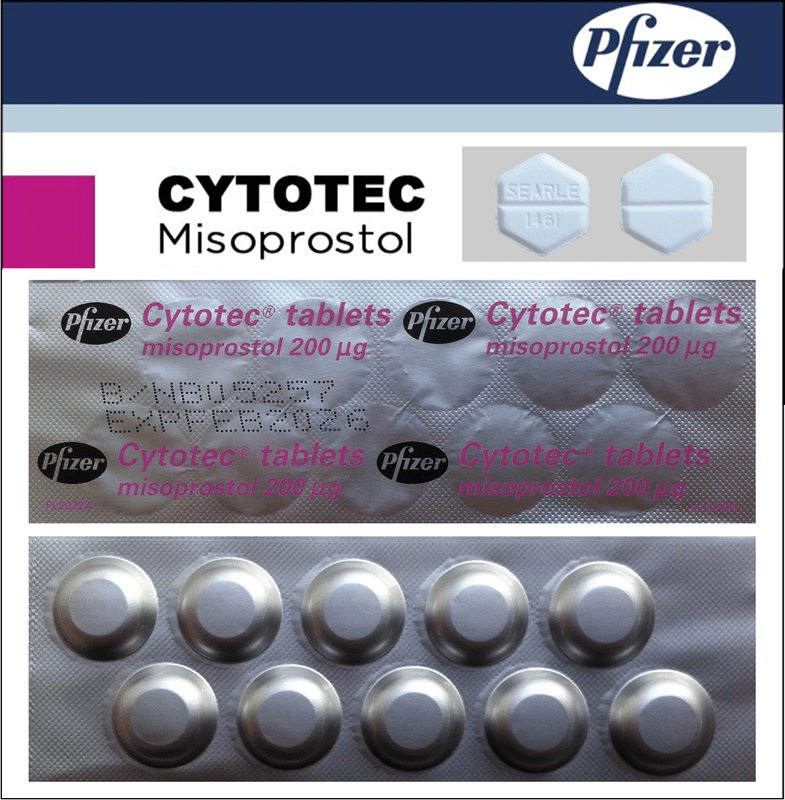
Cytotec
200 µg (200 mcg), tablets
Misoprostol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Cytotec and what is it used for
- 2. Important information before taking Cytotec
- 3. How to take Cytotec
- 4. Possible side effects
- 5. How to store Cytotec
- 6. Contents of the pack and other information
1. WHAT IS CYTOTEC AND WHAT IS IT USED FOR
Cytotec contains the active substance misoprostol. Misoprostol is similar to substances naturally produced in the body, called prostaglandins (in particular prostaglandin E1), which are produced, among other things, in the stomach, duodenum, and intestines.
Cytotec is indicated for the prevention of gastric and duodenal ulcers, which occur during treatment with non-steroidal anti-inflammatory drugs (NSAIDs).
2. IMPORTANT INFORMATION BEFORE TAKING CYTOTEC
When not to take Cytotec
- if the patient is allergic to misoprostol, other prostaglandins, or any of the other ingredients of this medicine (listed in section 6);
- if the patient is of childbearing age and does not use effective contraception to prevent pregnancy (for more information, see the "Pregnancy" section);
- if the patient is pregnant, trying to become pregnant, or has not had a negative pregnancy test result, as taking this medicine may cause miscarriage (for more information, see the "Pregnancy" section);
- during breastfeeding.
The patient should inform their doctor if they have heart or blood vessel diseases, including coronary artery disease, hypertension, or cerebral vascular diseases.
Warnings and precautions
Before starting to take Cytotec, the patient should discuss it with their doctor or pharmacist if:
- the patient is pregnant or plans to become pregnant (see the "Pregnancy" section below); due to the risk to the fetus, Cytotec should be stopped immediately;
- the patient is of childbearing age (see the "Pregnancy" section below); due to the risk to the fetus, it is essential to use effective contraception while taking Cytotec.
When to exercise special caution when taking Cytotec
- in women before menopause, unless the patient requires non-steroidal anti-inflammatory drugs (NSAIDs) and is at high risk of complications from these drugs. In such cases, effective methods of preventing pregnancy should be used,
- in patients with conditions predisposing to diarrhea, such as non-specific inflammatory bowel diseases. To reduce the risk of diarrhea, misoprostol should be taken with food and antacids containing magnesium should be avoided,
- in patients in whom dehydration may be hazardous. Such patients should be closely monitored.
In patients treated with NSAIDs and taking Cytotec, ulcers, perforations, and gastrointestinal bleeding may occur.
The doctor may decide to perform an endoscopy or biopsy before starting treatment to rule out cancer of the upper gastrointestinal tract.
Improvement of symptoms after taking Cytotec does not rule out the presence of a malignant change in the stomach.
Taking Cytotec in elderly patients (over 65 years old)
No dose adjustment is necessary.
Taking Cytotec in patients with renal impairment
No dose adjustment is necessary.
Taking Cytotec in patients with hepatic impairment
The effect of hepatic impairment on the metabolism of the medicine and its concentration in the blood is minimal.
Cytotec and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription. Studies have not shown any effect of Cytotec on the use of other medicines.
While taking Cytotec, the patient should avoid taking antacids containing magnesium, as they may exacerbate the diarrhea caused by misoprostol.
In rare cases, concomitant administration of NSAIDs and Cytotec may cause increased aminotransferase activity and peripheral edema.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking this medicine.
Pregnancy
If the patient is pregnant or plans to become pregnant, they should not take Cytotec.
If the patient becomes pregnant while taking Cytotec, the doctor will inform them about the risks associated with the treatment, as Cytotec may cause miscarriage, premature birth, or birth defects in the fetus. Pregnancies exposed to misoprostol in the first trimester were associated with approximately a 3-fold increased risk of birth defects in the fetus, particularly facial paralysis, limb defects, and developmental brain and skull defects. If the patient is exposed to Cytotec during pregnancy, they should discuss it with their doctor. If the patient decides to continue the pregnancy, they should be closely monitored until birth and undergo repeated ultrasound examinations, with particular attention to the fetus's limbs and head.
Cytotec should not be used during breastfeeding.
Cytotec should not be used during breastfeeding, as its metabolites pass into human milk, which may cause side effects in breastfed infants (e.g., diarrhea).
Driving and using machines
Cytotec may cause dizziness, so it may affect the ability to drive and use machines.
Cytotec contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means it is considered "sodium-free".
3. HOW TO TAKE CYTOTEC
This medicine should always be taken exactly as prescribed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
The recommended dose is from 400 micrograms to 800 micrograms per day in two or four divided doses. While taking Cytotec, the patient should continue to take non-steroidal anti-inflammatory drugs (NSAIDs) as prescribed by the doctor. If indicated, Cytotec should be taken at the same time as NSAIDs, until the end of NSAID therapy. Individual doses of Cytotec should be taken during breakfast and (or) main meals and before bedtime.
Taking a higher dose of Cytotec than recommended
In case of taking a higher dose of Cytotec than recommended, side effects listed below may occur, or existing side effects may worsen. If a higher dose of Cytotec than recommended is taken, the patient should immediately consult their doctor or pharmacist.
Missing a dose of Cytotec
A double dose should not be taken to make up for a missed dose.
Stopping Cytotec
In case of any further doubts about taking this medicine, the patient should consult their doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Cytotec can cause side effects, although not everybody gets them.
If any of the side effects get worse, or if the patient experiences any side effects not listed in this leaflet, they should tell their doctor or pharmacist.
Very common(may affect more than 1 in 10 people)
diarrhea*, rash
Common(may affect up to 1 in 10 people)
dizziness of central origin, headache, abdominal pain*, constipation, indigestion, bloating, nausea, vomiting, birth defects (fetal developmental abnormalities). If the patient becomes pregnant during treatment, they should stop taking Cytotec immediately and seek medical advice.
Uncommon(may affect up to 1 in 100 people)
vaginal bleeding (including bleeding in postmenopausal women), intermenstrual bleeding, menstrual disorders, uterine contractions, fever
Rare(may affect up to 1 in 1,000 people)
menstrual bleeding, painful menstruation, uterine rupture (uterine tear) after administration of prostaglandins in the second or third trimester of pregnancy (mainly in women who have had a previous delivery or have cesarean section scars). The patient should seek immediate medical attention.
Frequency not known(cannot be estimated from the available data)
anaphylactic reactions, amniotic fluid embolism, abnormal uterine contractions, fetal death, incomplete miscarriage, premature birth, retained placenta, uterine rupture, uterine perforation, uterine hemorrhage, chills.
*Diarrhea and abdominal pain were dose-related, usually occurred in the early stages of treatment, and were self-limiting. In rare cases, severe diarrhea has been reported, leading to severe dehydration.
Selected population groups
No special differences in the safety profile of misoprostol have been found in patients aged 65 years or older compared to younger patients.
The use of misoprostol in children has not been evaluated.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: + 48 (22) 49 21 301
fax: + 48 (22) 49 21 309
website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be gathered on the safety of the medicine.
5. HOW TO STORE CYTOTEC
Store in a temperature below 30°C.
The medicine should be stored out of sight and reach of children.
Do not use Cytotec after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Cytotec contains
- The active substance is misoprostol. One tablet contains 200 µg (200 mcg) of misoprostol.
- The other ingredients are: microcrystalline cellulose, sodium carboxymethylcellulose, hydrogenated castor oil, hypromellose.
What Cytotec looks like and contents of the pack
White or grayish-white hexagonal tablets with a score line on both sides, with the inscription "SEARLE 1461" embossed on one side.
Aluminum foil blisters in a cardboard box, containing 28 or 42 tablets.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Greece, the country of export:
Pfizer Hellas A.E.
243, Messoghion Ave
Neo Psychiko 154 51
Greece
Manufacturer:
Piramal Healthcare UK Limited
Whalton Road, Morpeth, Northumberland
NE61 3YA
United Kingdom
Piramal Pharma Solutions (Dutch) B.V.
Level, 7e verdieping, Bargelaan 200
2333 CW, Leiden
Netherlands
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Greek marketing authorization number, country of export: 40410/07/19-05-2008
Parallel import authorization number: 357/19
Date of leaflet approval: 17.09.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Pfizer Hellas A.E.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CitotecDosage form: Tablets, 200 mcgActive substance: misoprostolPrescription requiredDosage form: Tablets, 200 mcgActive substance: misoprostolPrescription requiredDosage form: Tablets, 200 mcgActive substance: misoprostolPrescription required
Alternatives to Citotec in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Citotec in Ukraine
Alternative to Citotec in Spain
Online doctors for Citotec
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Citotec – subject to medical assessment and local rules.














