
Citotec

Ask a doctor about a prescription for Citotec

How to use Citotec
PATIENT INFORMATION LEAFLET: USER INFORMATION
Cytotec, 200 µg, tablets
Misoprostol
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Cytotec and what is it used for
- 2. Important information before taking Cytotec
- 3. How to take Cytotec
- 4. Possible side effects
- 5. How to store Cytotec
- 6. Contents of the pack and other information
1. WHAT IS CYTOTEC AND WHAT IS IT USED FOR
Cytotec contains the active substance misoprostol. Misoprostol is similar to substances naturally produced in the body, called prostaglandins (in particular prostaglandin E1), which are produced, among other things, in the stomach, duodenum, and intestines.
Cytotec is indicated for the prevention of gastric and duodenal ulcers, which occur during treatment with non-steroidal anti-inflammatory drugs (NSAIDs).
2. IMPORTANT INFORMATION BEFORE TAKING CYTOTEC
When not to take Cytotec
- if you are allergic to misoprostol, other prostaglandins, or any of the other ingredients of this medicine (listed in section 6);
- if you are of childbearing age and do not use effective contraception to prevent pregnancy (for more information, see the section on "Pregnancy");
- if you are pregnant, trying to become pregnant, or have not had a negative pregnancy test result, as taking this medicine may cause miscarriage (for more information, see the section on "Pregnancy");
- during breastfeeding. You should inform your doctor if you have heart or blood vessel diseases, including coronary artery disease, high blood pressure, and cerebrovascular diseases.
Warnings and precautions
Before taking Cytotec, you should discuss it with your doctor or pharmacist if:
- you are pregnant or planning to become pregnant (see the section on "Pregnancy" below); due to the risk to the fetus, Cytotec should be stopped immediately;
- you are of childbearing age (see the section on "Pregnancy" below); due to the risk to the fetus, it is essential to use effective contraception while taking Cytotec.
When to be particularly careful when taking Cytotec
- in women before menopause, unless the patient requires non-steroidal anti-inflammatory drugs (NSAIDs) and is at high risk of complications from ulcers caused by these drugs. In such cases, effective methods of preventing pregnancy should be used,
- in patients with conditions predisposing to diarrhea, such as non-specific inflammatory bowel diseases. To reduce the risk of diarrhea, misoprostol should be taken with food and antacids containing magnesium should be avoided,
- in patients in whom dehydration may be hazardous. Such patients should be closely monitored.
In patients treated with NSAIDs and taking Cytotec, ulcers, perforations, and gastrointestinal bleeding may occur.
Your doctor may decide to perform an endoscopy or biopsy before starting treatment to rule out cancer of the upper gastrointestinal tract.
Improvement of symptoms after taking Cytotec does not exclude the presence of a malignant change in the stomach.
Taking Cytotec in elderly patients (over 65 years)
No dose adjustment is necessary.
Taking Cytotec in patients with renal impairment
No dose adjustment is necessary.
Taking Cytotec in patients with hepatic impairment
The effect of hepatic impairment on the metabolism and plasma concentration of the medicine is small.
Cytotec and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take, including those obtained without a prescription.
Studies have not shown any effect of Cytotec on the use of other medicines.
While taking Cytotec, you should avoid taking antacids containing magnesium, as they may increase the diarrhea caused by misoprostol.
In rare cases, concomitant administration of NSAIDs and Cytotec may cause increased aminotransferase activity and peripheral edema.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
If you are pregnant or plan to have a child, you should not take Cytotec.
If you become pregnant while taking Cytotec, your doctor will inform you about the risks associated with the treatment, as Cytotec may cause miscarriage, premature birth, or birth defects in the fetus. Pregnancies exposed to misoprostol in the first trimester were associated with an approximately 3-fold increased risk of birth defects in the fetus, particularly facial paralysis, limb defects, and developmental brain and skull defects. If you are exposed to Cytotec during pregnancy, you should discuss it with your doctor. If you decide to continue the pregnancy, you should be closely monitored until delivery and undergo repeated ultrasound examinations, with particular attention to the fetus's limbs and head.
Cytotec should not be used during breastfeeding.
Cytotec should not be used during breastfeeding, as its metabolites pass into human milk, which may cause side effects in breastfed infants (e.g., diarrhea).
Driving and using machines
Cytotec may cause dizziness, so Cytotec may affect your ability to drive and use machines.
Cytotec contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per tablet, which means it is essentially "sodium-free".
3. HOW TO TAKE CYTOTEC
This medicine should always be taken exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
The recommended dose is from 400 micrograms to 800 micrograms per day in two or four divided doses. While taking Cytotec, you should continue to take non-steroidal anti-inflammatory drugs (NSAIDs) as directed by your doctor. If indicated, Cytotec should be taken at the same time as NSAIDs until the end of NSAID therapy. Individual doses of Cytotec should be taken at mealtimes and (or) main meals and before bedtime.
Taking a higher dose of Cytotec than recommended
If a higher dose of Cytotec is taken than recommended, the side effects listed below may occur, or existing side effects may worsen. If you take more Cytotec than you should, contact your doctor or pharmacist immediately.
Missing a dose of Cytotec
Do not take a double dose to make up for a forgotten dose.
Stopping Cytotec
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Very common(may affect more than 1 in 10 people)
diarrhea*, rash
Common(may affect up to 1 in 10 people)
dizziness of central origin, headache, abdominal pain*, constipation, indigestion, bloating, nausea, vomiting, birth defects (fetal developmental abnormalities). If you become pregnant during treatment, you should stop taking Cytotec immediately and seek medical advice.
Uncommon(may affect up to 1 in 100 people)
vaginal bleeding (including bleeding in postmenopausal women), intermenstrual bleeding, menstrual disorders, uterine contractions, fever
Rare(may affect up to 1 in 1,000 people)
menstrual bleeding, painful menstruation, uterine rupture (uterine tear) after administration of prostaglandins in the second or third trimester of pregnancy (mainly in women who have had a previous delivery or have cesarean section scars). You should seek immediate medical attention.
Frequency not known(cannot be estimated from the available data)
anaphylactic reactions, amniotic fluid embolism, abnormal uterine contractions, fetal death, incomplete miscarriage, premature birth, retained placenta, uterine rupture, uterine perforation, uterine hemorrhage, chills.
* Diarrhea and abdominal pain were dose-related, usually occurred in the early stages of treatment, and were self-limiting. In rare cases, severe diarrhea has been reported, leading to severe dehydration.
Selected population groups
No special differences in the safety profile of misoprostol have been observed in patients over 65 years of age compared to younger patients.
The use of misoprostol in children has not been evaluated.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please tell your doctor, pharmacist, or nurse. You can also report side effects directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE CYTOTEC
Store in a temperature below 25°C, protected from light.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after (EXP). The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Cytotec contains
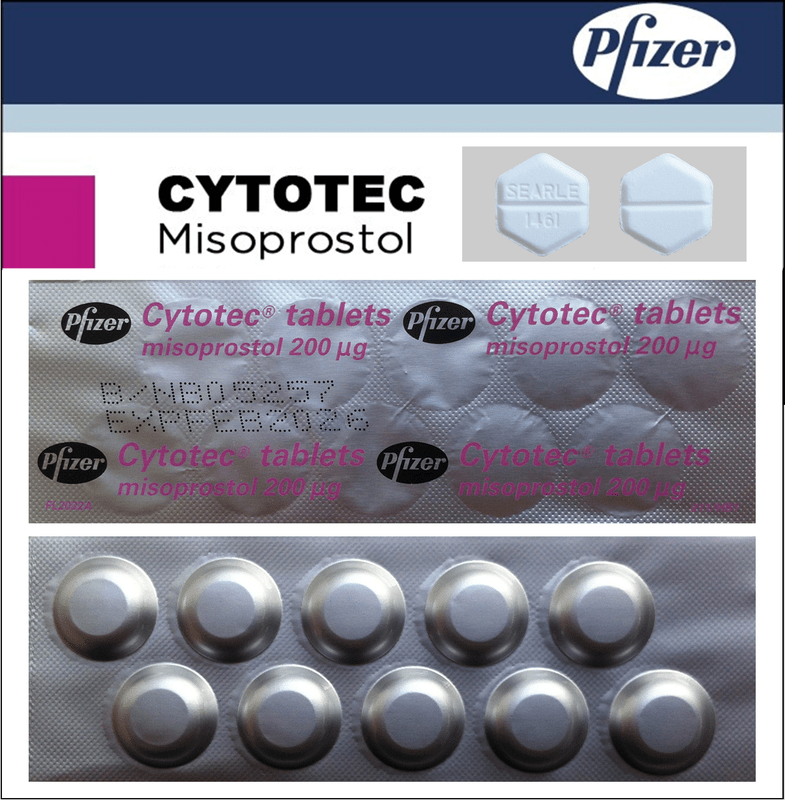
- The active substance is misoprostol (Misoprostolum). One tablet contains 200 µg (micrograms) of misoprostol.
- The other ingredients are: microcrystalline cellulose, sodium starch glycolate (see section 2 "Cytotec contains sodium"), hydrogenated castor oil, hypromellose.
What Cytotec looks like and contents of the pack
White or grayish hexagonal tablets with a score line on both sides, with the inscription "SEARLE 1461" embossed on one side.
Aluminum foil blisters in a cardboard box, containing 30 or 50 tablets.
Marketing authorization holder
Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium
Importer
Piramal Pharma Solutions (Dutch) B.V., Bargelaan 200, 2333 CW, Leiden, Netherlands
To obtain more detailed information, please contact the representative of the marketing authorization holder:
Pfizer Polska Sp. z o.o., tel.: 22 335 61 00
Date of leaflet approval:07/2024
Detailed and up-to-date information about this product can be obtained by scanning the QR code on the outer packaging using a mobile device. The same information is also available at the URL: https://pfi.sr/ulotka-cytotec and on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products http://www.urpl.gov.pl.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterPiramal Pharma Solutions (Dutch) B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CitotecDosage form: Tablets, 200 mcgActive substance: misoprostolPrescription requiredDosage form: Tablets, 200 mcgActive substance: misoprostolPrescription requiredDosage form: Tablets, 200 mcgActive substance: misoprostolPrescription required
Alternatives to Citotec in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Citotec in Ucrania
Alternative to Citotec in España
Online doctors for Citotec
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Citotec – subject to medical assessment and local rules.














