
Curosurf

Ask a doctor about a prescription for Curosurf

How to use Curosurf
Leaflet attached to the packaging: information for parents and guardians
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Curosurf, 80 mg/ml, suspension for intratracheal and intra-bronchial use
(Poractant alfa)
Fraction of phospholipids from pig lungs
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, you should tell the doctor or nurse. See section 3.
Table of contents of the leaflet:
- 1. What is Curosurf and what is it used for
- 2. How to use Curosurf
- 3. Possible side effects
- 4. How to store Curosurf
- 5. Contents of the packaging and other information
1. WHAT IS CUROSURF AND WHAT IS IT USED FOR
Curosurf is used to treat and prevent respiratory distress syndrome (RDS) in newborns. In most newborns, a substance called surfactant (surface-active agent) is present in the lungs. This substance covers the alveoli, prevents them from sticking together, and allows for normal breathing. However, some newborns, especially premature babies, are born with a surfactant deficiency, which leads to RDS. Curosurf is a natural surfactant that works in the same way as the surfactant produced by newborns, and therefore helps newborns breathe normally until they start producing their own natural surfactant.
Newborns may have other conditions that require different treatment.
2. HOW TO USE CUROSURF
Dosage:
The doctor will choose the appropriate dose of the medicine, depending on the child's weight. If the child is given Curosurf to prevent RDS, the medicine should be administered within 15 minutes of birth.
If the child is given Curosurf to treat RDS, the medicine should be administered as soon as possible after the condition is diagnosed. If the child needs an additional dose of Curosurf, it is given 12 hours after the first dose. If necessary, a third dose can be given after another 12 hours.
The use of Curosurf in premature babies with liver or kidney failure has not been studied.
Method of administration:
Curosurf is administered to the child in an incubator by a doctor or nurse. The medicine is warmed to room temperature and then administered using a syringe through an endotracheal tube into the child's trachea. This may require disconnecting the child from the ventilator for a few minutes.
A less invasive method of surfactant administration through a thin catheter (LISA - Less Invasive Surfactant Administration) may also be used.
Curosurf contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per vial, which means that the medicine is considered "sodium-free".
3. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Possible side effects are listed below by frequency of occurrence:
In case of doubts about side effects, you should consult a doctor.
Uncommon(occurring in less than 1 in 100 patients):
- infection
- bleeding into the brain
- air in the chest cavity caused by lung damage
Rare(occurring in less than 1 in 1000 patients):
- slow heart rate
- low blood pressure
- chronic lung disease
- decreased oxygen levels in the body
The following side effects have also been reported:
- increased oxygen levels in the body
- blue discoloration of the skin or gums due to oxygen deficiency
- respiratory arrest
- complications due to the insertion of tubes into the lungs
- decreased brain activity
During the administration of Curosurf through a thin catheter, some mild and short-term side effects have been reported: bradycardia, apnea, decreased oxygen saturation, foam on the mouth, coughing, choking, and sneezing.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell the doctor or nurse.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help gather more information on the safety of the medicine.
4. HOW TO STORE CUROSURF
- The medicine should be stored out of sight and out of reach of children.
- Store in a refrigerator (2°C - 8°C). Store in the original packaging to protect from light. Before administration to the child, warm to room temperature.
- Unopened and unused vials of Curosurf that have been warmed to room temperature can be returned to the refrigerator within 24 hours for later use. The medicine should not be warmed to room temperature and returned to the refrigerator more than once.
- Do not use this medicine after the expiry date stated on the carton and vial. The expiry date refers to the last day of the month stated.
- A single container should be used once, and any remaining contents should be discarded. The hospital should ensure the safe disposal of unused Curosurf.
- Medicines should not be disposed of via wastewater. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
5. CONTENTS OF THE PACKAGING AND OTHER INFORMATION
What Curosurf contains
- The active substance is a mixture of lipids and proteins obtained from pig lung alveoli.
- The other ingredients are: sodium chloride, water for injections, sodium bicarbonate (for pH adjustment).
What Curosurf looks like and what the packaging contains
Curosurf is a sterile suspension. It is available in single-dose glass vials containing 1.5 ml (120 mg) of phospholipid fraction from pig lung alveoli.
Each ml of sterile suspension contains 80 mg of phospholipid fraction from pig lung alveoli. One packaging contains 2 vials of 1.5 ml Curosurf suspension.
To obtain more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Bulgaria, the country of export:
Chiesi Farmaceutici S.p.A.
Via Palermo, 26/A
43122 Parma, Italy
Manufacturer:
Chiesi Farmaceutici S.p.A
Via San Leonardo 96 - Via Palermo 26/A, 43122 Parma, Italy
Chiesi Pharmaceuticals GmbH
Gonzagagasse 16/16, A-1010 Vienna, Austria
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Authorization number in Bulgaria, the country of export: 9600101
Parallel import authorization number: 211/24
Date of leaflet approval: 24.05.2024
[Information about the trademark]
Please read the information on the back of the leaflet
------------------------------------------------------------------------------------------------------------------------
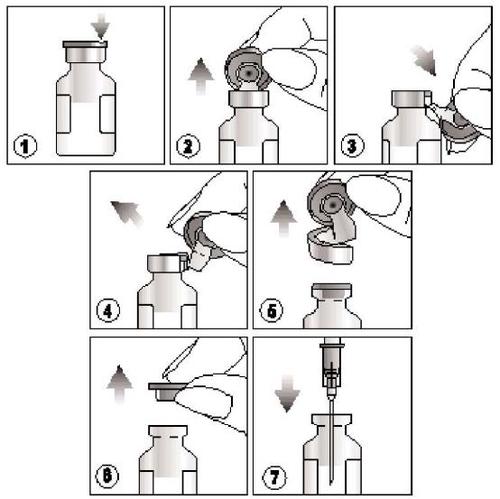
How to open the product?
- 1) Find the notch on the colored, plastic cap,
- 2) Lift the cap at the notch and pull it upwards,
- 3) Pull down the plastic part of the cap along with its aluminum part,
- 4) and 5) Remove the entire cap by pulling the aluminum ring
- 6) and 7) Remove the rubber stopper before withdrawing the contents of the vial.
For single use only. Any unused suspension remaining in the vial should be discarded.
Do not store unused suspension for later use.
Any remaining product or waste should be disposed of in accordance with the regulations.
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Chiesi Farmaceutici S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CurosurfDosage form: Suspension, 80 mg, corresponding to approximately 74 mg of total phospholipid content and 0.9 mg of low molecular weight hydrophobic proteins.Active substance: natural phospholipidsManufacturer: Chiesi Farmaceutici S.p.A. Chiesi Pharmaceuticals GmbHPrescription not requiredDosage form: Suspension, 80 mg/mlActive substance: natural phospholipidsPrescription not requiredDosage form: Lozenge, 1500 mg + 125 mgActive substance: nikethamideManufacturer: Przedsiębiorstwo Produkcyjno-Handlowe EWA S.A.Prescription not required
Alternatives to Curosurf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Curosurf in Ukraine
Alternative to Curosurf in Spain
Online doctors for Curosurf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Curosurf – subject to medical assessment and local rules.














