
Cluvot 1250 i.m.
Ask a doctor about a prescription for Cluvot 1250 i.m.

How to use Cluvot 1250 i.m.
Leaflet accompanying the packaging: patient information
Cluvot 1250 IU
Powder and solvent for solution for injection/infusion
Human blood coagulation factor XIII
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Cluvot and what is it used for
- 2. Important information before using Cluvot
- 3. How to use Cluvot
- 4. Possible side effects
- 5. How to store Cluvot
- 6. Contents of the packaging and other information
1. What is Cluvot and what is it used for
What is Cluvot
Cluvot is a medicine that comes as a white powder and solvent. The reconstituted solution should be administered by intravenous injection.
Cluvot is human blood coagulation factor XIII (F XIII) produced from human plasma (the liquid part of the blood) and plays an important role in the process of haemostasis (stopping bleeding).
What is Cluvot used for
Cluvot is indicated for use in adults, children and adolescents.
- for prophylactic treatment of congenital factor XIII deficiency and
- in perioperative management to treat bleeding during surgical procedures in patients with congenital factor XIII deficiency.
2. Important information before using Cluvot
This section of the leaflet contains information that you should consider before starting to use Cluvot.
When not to use Cluvot:
- if you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
Tell your doctor if you have any hypersensitivity to any medicine or food.
Warnings and precautions:
- if you have had allergic reactions to factor XIII in the past. As a precaution, antihistamines and corticosteroids should be used if the doctor decides to do so.
- if you experience any signs of allergic reactions or anaphylactic reactions (severe allergic reactions can cause significant difficulty breathing or dizziness). Administration of Cluvot should be stopped immediately (i.e. injection or infusion should be discontinued). In case of shock, treatment should be administered according to current medical standards.
- if you have had recent thrombosis (blood clot). You should be carefully monitored due to the stabilizing effect of F XIII on fibrin.
- the formation of inhibitors (antibodies that neutralize) is known as a complication of treatment and means that the treatment stops working. If bleeding is not controlled with Cluvot, you should inform your doctor immediately. You should be closely monitored for the development of inhibitors.
Your doctor should always weigh the benefits of treatment against the risk of complications.
Virus safety
When medicines are made from human blood or plasma, various measures are taken to protect patients from the transmission of infectious agents. These measures include:
- careful selection of blood and plasma donors to exclude the risk of transmission of infectious agents
- testing of each donation and plasma pool for the presence of virus markers/infections.
- introduction of steps in the manufacturing process that can inactivate or remove viruses.
Despite the implementation of these measures, it cannot be completely excluded that the transmission of infectious agents may occur when administering a medicinal product derived from human blood or plasma. This risk also applies to unknown or newly discovered viruses and other infectious agents.
The measures taken are effective against enveloped viruses such as human immunodeficiency virus (HIV, the virus that causes AIDS), hepatitis B virus (HBV, which causes hepatitis B), hepatitis C virus (HCV, which causes hepatitis C), and non-enveloped viruses such as hepatitis A virus (HAV, which causes hepatitis A) and parvovirus B19.
For patients receiving repeated doses of products derived from human plasma, consideration should be given to vaccination against hepatitis A and B.
It is recommended that the date of administration, batch number, and volume administered be recorded each time Cluvot is administered.
Cluvot and other medicines
- Tell your doctor or pharmacist about any other medicines you are taking, including those available without a prescription.
- No interactions between factor XIII concentrate and other medicinal products are known.
- Cluvot should not be mixed with other medicinal products, solvents, or diluents, except for those mentioned in section 6, and should be administered using separate infusion sets.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before using this medicine.
- Limited data on the clinical use of Cluvot in pregnancy do not indicate any negative effects on pregnancy and embryonic and foetal development. Cluvot may be used during pregnancy if necessary.
- The extent of penetration of Cluvot into human milk is unknown, but considering its high molecular weight, the likelihood of excretion into breast milk is very low, and due to its protein nature, absorption of intact particles by the newborn is also unlikely. Therefore, Cluvot can be used during breastfeeding.
- No data are available on the effect of Cluvot on fertility.
Driving and using machines
No studies have been conducted on the effects on the ability to drive and use machines.
Important information about some of the ingredients of Cluvot
Cluvot contains sodium.
Note that Cluvot contains sodium. This is important for patients on a controlled low-sodium diet. Cluvot contains 124.4 to 195.4 mg (5.41 to 8.50 mmol) of sodium per dose (40 IU/kg body weight - assuming an average body weight of 70 kg), if the recommended dose (2800 IU = 44.8 ml) is administered.
3. How to use Cluvot
- Cluvot is usually administered by a doctor.
- Cluvot is intended for intravenous administration only.
Dosage
Your doctor will calculate the appropriate dose and decide how often Cluvot should be administered to you, taking into account your progress.
More detailed recommendations can be found in the section "Information intended for healthcare professionals only".
Overdose
No cases of overdose have been reported, and none are expected when the medicine is administered by medical personnel.
4. Possible side effects
Like all medicines, Cluvot can cause side effects, although not everybody gets them.
The following side effects have been observed rarely(in more than 1 in 10,000 patients and less than 1 in 1,000)
- Allergic reactions such as generalized urticaria (itchy swelling of the skin), rash, decreased blood pressure (which can cause dizziness or fainting), difficulty breathing.
- Increased body temperature
The following side effects have been observed very rarely(in less than 1 in 10,000 patients):
- Formation of inhibitors of FXIII.
If allergic reactionsoccur, administration of Cluvot should be stopped immediately and appropriate treatment should be started. Current medical standards should be used to treat shock.
Side effects in children and adolescents
It is expected that side effects in children are the same as in adults.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C,
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Cluvot
- Store in a refrigerator (2°C - 8°C).
- Do not freeze.
- Store in the outer packaging to protect from light.
- Cluvot does not contain preservatives. The medicine should be used immediately after reconstitution. If it is not used immediately, storage at room temperature should not exceed 4 hours. Do not store in a refrigerator and do not freeze the solution after reconstitution.
- The medicine should be stored out of sight and reach of children.
- Do not use Cluvot after the expiry date stated on the label and outer packaging after the abbreviation EXP.
- The batch number of the medicinal product is stated on the outer packaging and label after the abbreviation: Lot.
6. Contents of the packaging and other information
What Cluvot contains Active substance:
Human blood coagulation factor XIII (FXIII) concentrate containing 1250 IU per vial.
Excipients:
Human albumin, glucose monohydrate, sodium chloride, sodium hydroxide (in small amounts to adjust pH).
Solvent:Water for injections
What Cluvot looks like and contents of the pack
Cluvot comes as a white powder and solvent, which is water for injections. The reconstituted solution should be colourless, clear to slightly opalescent. When held up to the light, it should not be cloudy or contain particles.
Pack sizes
One pack of 1250 IU contains:
1 vial of powder
1 vial of 20 ml water for injections
1 Mix2Vial transfer system with filter
Administration set (inner packaging);
1 disposable 20 ml syringe
1 injection set
2 alcohol swabs
1 non-sterile plaster
Marketing authorization holder and manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg
Germany
Date of last revision of the leaflet:August 2021
----------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only: Dosage
1 ml is approximately equal to 62.5 IU, and 100 IU is equivalent to 1.6 ml.
Important:
The amount to be administered and the frequency of administration should always be adjusted according to the individual patient's clinical efficacy.
Dosage
Dosage should be individually tailored based on body weight, laboratory results, and the patient's clinical condition.
Routine dosing schedule for prophylaxis
Initial dose
- 40 international units (IU) per kilogram of body weight.
- The infusion rate should not exceed 4 ml per minute
Subsequent doses
- Dosage should be determined based on the current level of FXIII activity, and doses should be administered at 28-day intervals (4 weeks) to maintain a minimum FXIII activity level of approximately 5 to 20%.
- Recommended dose adjustment of +/- 5 IU/kg body weight should be calculated based on the minimum FXIII activity level as shown in Table 1 and the patient's clinical condition.
- Dose adjustment should be based on a specific, sensitive test used to determine FXIII levels. Examples of dose adjustment using the standard Berichrom activity test are presented in the following Table 1.
Table 1: Dose adjustment using the Berichrom activity test
Activity is expressed in units using the Berichrom activity test, referring to the current International Standard for Plasma Factor XIII. Therefore, one unit corresponds to one International Unit.
| Minimum FXIII activity level (%) | Dose adjustment |
| One minimum level <5% | Increase by 5 units/kg. |
| Minimum level 5% to 20% | No change |
| Two minimum levels > 20% | Decrease by 5 units/kg. |
| One minimum level > 25% | Decrease by 5 units/kg. |
Preoperative prophylaxis.
After the last dose administered in routine prophylaxis, in the case of a planned surgical procedure:
- Between 21 and 28 days after the last dose - a full dose should be administered to the patient immediately before surgery, and the next prophylactic dose should be administered 28 days later.
- Between 8 and 21 days after the last dose - an additional dose (full or partial) may be administered before surgery. The dose should be based on the patient's FXIII activity level, clinical condition, and should be adjusted according to the half-life of the medicinal product Cluvot.
- Within 7 days of the last dose - additional administration may not be necessary.
Dose adjustment may be different from the recommended dose and should be individually tailored based on FXIII activity level and the patient's clinical condition. All patients should be closely monitored during and after surgery.
Therefore, it is recommended to monitor the increase in FXIII activity level based on the FXIII test. In the case of major surgical procedures and significant bleeding, the goal should be to achieve near-normal values (healthy individuals: 70%-140%).
Children and adolescents
Dosage and administration in children and adolescents are based on body weight and generally do not differ from the guidelines for adults. Dosage and/or administration frequency for each patient should always be adjusted based on clinical efficacy and FXIII activity level.
Elderly patients
Dosage and administration in elderly patients (> 65 years) have not been documented in clinical trials.
Method of administration
General instructions
The solution should be clear or slightly opalescent. After filtration/removal of the vial contents (see below), the reconstituted product before administration should be visually inspected; check for any contamination or change in colour.
Do not use cloudy solutions or those containing flakes or particles.
Reconstitution and removal from the vial must be performed under aseptic conditions.
Reconstitution
Bring the solvent to room temperature. Remove the plastic caps from the vials containing the powder and solvent, and wipe the stoppers with an aseptic solution. After drying, open the Mix2Vial system containing the connector.
1 |
|
2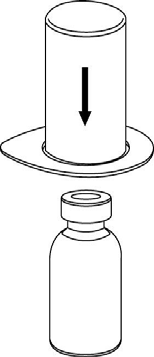 |
|
3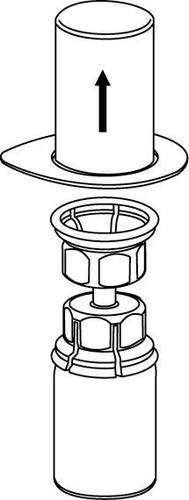 |
|
4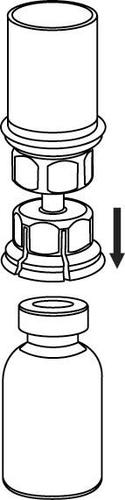 |
|
5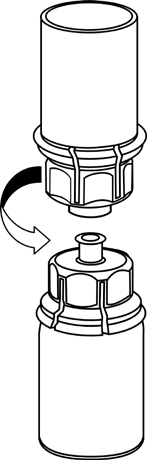 |
|
6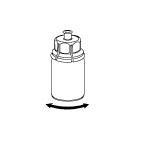 |
|
7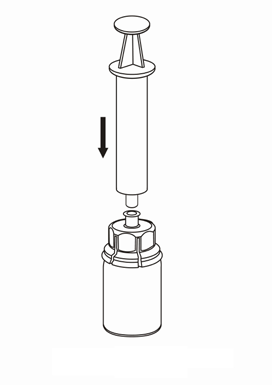 |
|
Withdrawal and administration
8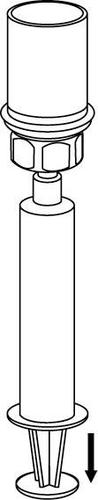 |
|
9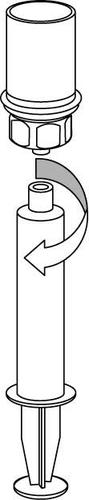 |
|
Caution should be exercised to prevent blood from entering the syringe containing the medicinal product, as there is a risk that the blood may clot in the syringe and fibrin clots could be administered to the patient.
The reconstituted solution should be administered through separate infusion sets (supplied with the product) by slow intravenous injection, at a rate not exceeding 4 ml per minute.
Any unused medicinal products and their waste should be disposed of in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterCSL Behring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Cluvot 1250 i.m.Dosage form: Powder, 250 IUActive substance: coagulation factor XIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 1000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 2000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription required
Alternatives to Cluvot 1250 i.m. in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cluvot 1250 i.m. in Spain
Online doctors for Cluvot 1250 i.m.
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cluvot 1250 i.m. – subject to medical assessment and local rules.














