
Clemastinum Vzf

Ask a doctor about a prescription for Clemastinum Vzf

How to use Clemastinum Vzf
Package Leaflet: Information for the Patient
CLEMASTINUM WZF, 1 mg/ml, Solution for Injection
Clemastinum
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Clemastinum WZF and what is it used for
- 2. Important information before using Clemastinum WZF
- 3. How to use Clemastinum WZF
- 4. Possible side effects
- 5. How to store Clemastinum WZF
- 6. Contents of the pack and other information
1. What is Clemastinum WZF and what is it used for
Clemastinum WZF is an antiallergic medicine that relieves allergic symptoms, used in adults:
- as an adjunctive treatment in anaphylactic shock and angioedema (symptoms: low blood pressure, skin swelling, lips, tongue, throat swelling, causing shortness of breath);
- before a procedure that may cause histamine release in the body, to prevent severe allergic reactions.
2. Important information before using Clemastinum WZF
When not to use Clemastinum WZF
- if you are taking monoamine oxidase inhibitors (MAOIs) - see "Clemastinum WZF and other medicines".
Warnings and precautions
Before starting treatment with Clemastinum WZF, discuss it with your doctor or pharmacist:
Clemastinum WZF and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
- Taking some medicines with clemastine may affect the central nervous system, especially if you are taking:
- barbiturates (sleeping pills and sedatives);
- tricyclic antidepressants (used to treat depression);
- medicines used in parkinsonism;
- strong painkillers (opioids, e.g., morphine).
- Some antidepressants (monoamine oxidase inhibitors - MAOIs) prolong and enhance the effect of clemastine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
The medicine should only be used in pregnancy if absolutely necessary. The doctor will decide whether you can use Clemastinum WZF during pregnancy.
Do not use the medicine while breastfeeding, as clemastine passes into breast milk in small amounts and may cause side effects in breastfed infants.
Driving and using machines
Due to the possibility of side effects (e.g., drowsiness, fatigue, dizziness), do not drive or operate machinery while taking the medicine.
Clemastinum WZF contains sorbitol, ethanol 96%, propylene glycol, and sodium
The medicine contains 90 mg of sorbitol in 2 ml of solution.
Sorbitol is a source of fructose. If you have previously been diagnosed with hereditary fructose intolerance, a rare genetic disorder, you should not take this medicine. In patients with hereditary fructose intolerance, the body does not break down the fructose contained in this medicine, which may cause severe side effects.
Tell your doctor before taking this medicine if you have hereditary fructose intolerance.
This medicine contains 140 mg of alcohol (ethanol 96%) in 2 ml of solution (ampoule). The amount of alcohol in 2 ml of this medicine is equivalent to less than 3 ml of beer and about 1 ml of wine.
A small amount of alcohol in this medicine will not cause noticeable effects.
The medicine contains 600 mg of propylene glycol in 2 ml of solution.
The medicine contains less than 1 mmol (23 mg) of sodium in 2 ml of solution, which means the medicine is considered "sodium-free".
The medicine can be diluted with 0.9% NaCl solution or 5% glucose solution. The sodium content from the diluent should be taken into account when calculating the total sodium content in the prepared dilution of the medicine. To obtain accurate information about the sodium content in the solution used to dilute the medicine, refer to the patient leaflet of the diluent used.
3. How to use Clemastinum WZF
Clemastinum WZF is usually administered by medical personnel.
The medicine is intended for adults. It should be administered intravenously or intramuscularly.
Duration of treatment
The duration of treatment is determined by the doctor - the medicine is used as needed.
Detailed dosing and administration instructions are provided at the end of the leaflet, in the section "Information intended exclusively for healthcare professionals".
Using a higher dose of Clemastinum WZF than recommended
The medicine is administered by medical personnel, so it is unlikely that you will receive a higher dose than recommended. Tell your doctor or nurse if you experience:
- excitement, hallucinations, confusion, coordination disorders, muscle tremors, involuntary movements, overheating, cyanosis, convulsions, increasing breathing difficulties;
- dry mouth, pupil dilation, facial flushing, elevated body temperature, drowsiness.
Missing a dose of Clemastinum WZF
The medicine is administered by medical personnel, so it is unlikely that a dose will be missed.
If you have any further questions about using the medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects are listed with their frequency of occurrence.
The most common ones are:
- sedation, drowsiness, coordination disorders, dizziness;
- abdominal pain, heartburn, nausea, vomiting, diarrhea, constipation;
- increased density of respiratory tract secretions. May occur:
- fatigue, confusion, anxiety, excessive stimulation (especially in children), weakness, headache, tremors, insomnia, blurred vision, double vision, tinnitus, convulsions;
- loss of appetite, dry mouth;
- feeling of chest tightness, wheezing, dryness of the nasal and throat mucosa, feeling of a blocked nose;
- low blood pressure, palpitations, accelerated heart rate, extra beats;
- changes in blood count (decreased platelet count, granulocytopenia in peripheral blood, hemolytic anemia);
- urination difficulties, urinary retention;
- hives, rash;
- excessive sweating, chills, hypersensitivity to light.
In patients over 60 years old, there is a higher likelihood of low blood pressure, drowsiness, fatigue, and dizziness.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Clemastinum WZF
Keep the medicine out of the sight and reach of children.
Store in a temperature below 25°C. Do not freeze. Store the ampoules in the outer packaging to protect from light.
Do not use this medicine after the expiry date stated on the carton and ampoule.
The expiry date stated is the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Clemastinum WZF contains
- The active substance of the medicine is clemastine. Each ml of solution contains 1 mg of clemastine (as clemastine fumarate).
- The other ingredients are: sorbitol, ethanol 96%, propylene glycol, sodium citrate, water for injections.
What Clemastinum WZF looks like and contents of the pack
Clemastinum WZF is a colorless, clear liquid.
Each pack of the medicine contains 5 ampoules of 2 ml, in a cardboard box.
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last revision of the leaflet:
Information intended exclusively for healthcare professionals:
CLEMASTINUM WZF, 1 mg/ml, Solution for Injection
Clemastinum
The medicine should be administered intravenously or intramuscularly.
Before intravenous administration, the contents of the ampoule should be diluted five times (1:5) with 0.9% NaCl solution (see section 2, subsection "Clemastinum WZF contains sorbitol, ethanol 96%, propylene glycol, and sodium") or 5% glucose solution. During preparation and administration of the medicine, aseptic rules must be followed. Inject slowly over 2-3 minutes.
Ampoule opening instructions
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to help the solution flow down.
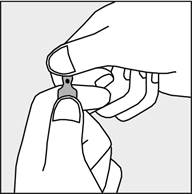
A colored dot is marked on each ampoule (see figure 1), as a sign of the break point located below it.
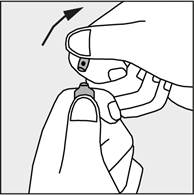
- To open the ampoule, hold it vertically in both hands with the colored dot facing each other - see figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
- Press in the direction of the arrow shown in figure 3. The ampoules are intended for single use only and should be opened immediately before use. Any remaining contents of unused medicine should be destroyed in accordance with applicable regulations. Figure 1 Figure 2 Figure 3
Adults
Adjunctive treatment in anaphylactic shock, angioedema:
intravenously or intramuscularly 2 ml (1 ampoule) twice a day.
Prophylactically, before a procedure that may cause histamine release:
intravenously 2 ml (1 ampoule) immediately before the procedure.
Children
The medicine is not recommended for use in children.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Clemastinum VzfDosage form: Syrup, 0.5 mg/5 mlActive substance: clemastineManufacturer: Aflofarm Farmacja Polska Sp. z o.o.Prescription requiredDosage form: Syrup, 1 mg/10 mlActive substance: clemastinePrescription requiredDosage form: Tablets, 1 mgActive substance: clemastinePrescription required
Alternatives to Clemastinum Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Clemastinum Vzf in Ukraine
Alternative to Clemastinum Vzf in Spain
Online doctors for Clemastinum Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Clemastinum Vzf – subject to medical assessment and local rules.














