

Cetix

Ask a doctor about a prescription for Cetix

How to use Cetix
Leaflet attached to the packaging: patient information
Cetix, 100 mg/5 ml, granules for oral suspension
Cefixime
Read the contents of the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Cetix and what is it used for
- 2. Important information before taking Cetix
- 3. How to take Cetix
- 4. Possible side effects
- 5. How to store Cetix
- 6. Contents of the packaging and other information
1. What is Cetix and what is it used for
Cetix contains the active substance cefixime. It belongs to a group of antibiotics called cephalosporins, which are used to treat bacterial infections.
Cetix is used in children over 6 months, adolescents, and adults to treat:
- middle ear infections;
- sinus infections;
- throat infections;
- infections that cause exacerbation of chronic bronchitis;
- severe community-acquired pneumonia;
- urinary tract infections.
2. Important information before taking Cetix
When not to take Cetix:
- if the patient is allergic to cefixime or any of the other ingredients of this medicine (listed in section 6),
- if the patient is allergic (hypersensitive) to other antibiotics in the cephalosporin group,
- if the patient has ever had a severe allergic reaction to penicillin or other beta-lactam antibiotics.
This medicine should not be given to premature babies or full-term newborns.
Do not take this medicine if you have any of the above conditions. Before starting treatment with Cetix oral suspension, consult a doctor or pharmacist.
Warnings and precautions
Before starting treatment with Cetix, discuss it with your doctor or pharmacist if:
- the patient has ever had colitis,
- the patient has kidney problems,
- the patient is a child under 6 months old.
If in doubt whether any of the above warnings apply to the patient, consult a doctor or pharmacist before starting treatment with this medicine.
Cetix is not suitable for every patient.
If the patient has any of the situations described below, they should tell their doctor before starting treatment with Cetix.
- The patient is allergic to penicillin or other beta-lactam antibiotics. An allergic reaction may include a rash, itching, difficulty swallowing or breathing, swelling of the face, lips, throat, or tongue. Not all people with a penicillin allergy are also allergic to cephalosporins. However, caution should be exercised if the patient has ever had an allergic reaction to penicillins, as they may also be allergic to Cetix.
- In patients who develop a severe allergic reaction or anaphylaxis (a severe allergic reaction that causes difficulty breathing or dizziness) after taking Cetix, the medicine should be discontinued and appropriate treatment should be administered.
- The patient is taking other medicines known to have harmful effects on the kidneys. If the patient has any kidney disease, they should also inform their doctor. The doctor may recommend regular tests to assess kidney function during treatment.
- The patient has severe or persistent diarrhea, which may be accompanied by stomach pain or cramps - these symptoms may occur during treatment with Cetix or shortly after its completion. In this case, the patient should stop taking the medicine and consult a doctor immediately. Do not take medicines that slow down or stop bowel movements.
If the patient experiences symptoms known as DRESS (Drug Hypersensitivity Syndrome - drug rash with eosinophilia and systemic symptoms) or Stevens-Johnson syndrome, or toxic epidermal necrolysis (see section 4. Possible side effects), they should stop taking Cetix and consult a doctor immediately.
During treatment with Cetix, the risk of infections caused by other types of bacteria that Cetix does not affect may increase for a while. For example, thrush (an infection caused by a yeast called Candida) may occur.
This medicine may cause vomiting and diarrhea (see section 4. Possible side effects). This may weaken the effectiveness of Cetix and/or other oral medicines (e.g., oral contraceptives).
Cetix and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
In particular, tell your doctor or pharmacist if you are taking:
- medicines known to have harmful effects on the kidneys:
- antibiotics, including aminoglycosides, colistin, polymyxin, and viomycin;
- diuretics, such as ethacrynic acid or furosemide, which increase the amount of urine produced by the body;
- nifedipine, a medicine used to treat high blood pressure or heart disease;
- anticoagulant medicines (blood thinners), such as warfarin, in some patients. Cefixime can cause blood clotting disorders and may prolong blood clotting time.
Effect on laboratory test results
If the patient is to undergo blood or urine tests, they should inform their doctor that they are taking Cetix, as cefixime may affect the results of some of these tests.
- Cetix may affect the results of some urine tests that measure sugar levels (e.g., Benedict's or Fehling's test). If the patient has diabetes and has their urine tested frequently, they should tell their doctor, as other tests may be needed to control sugar levels while taking this medicine.
- Cetix may affect the results of some tests that detect ketones in the urine. The patient should tell their doctor that they are taking Cetix, as other tests may be needed.
- Cetix may affect the results of blood tests that detect antibodies (the direct Coombs test).
Cetix with food and drink
Cetix can be taken with or without food.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor before taking this medicine.
Driving and using machines
Cetix usually does not affect the ability to drive or use machines.
Cetix contains sucrose
The medicine contains 2.52 g of sucrose in 5 ml of the ready-to-use suspension.
This should be taken into account in patients with diabetes. If the patient has previously been diagnosed with intolerance to some sugars, they should consult a doctor before taking this medicine.
Cetix contains sodium benzoate (E211)
The medicine contains 2.5 mg of sodium benzoate (E211) in 5 ml of the ready-to-use suspension. Sodium benzoate may increase the risk of jaundice (yellowing of the skin and whites of the eyes) in newborns (up to 4 weeks of age).
The medicine contains less than 1 mmol (23 mg) of sodium in 5 ml of the ready-to-use suspension, which means it is considered "sodium-free".
3. How to take Cetix
This medicine should always be taken as directed by a doctor or pharmacist. In case of doubts, consult a doctor or pharmacist.
The prescribed dose by the doctor depends on the type and severity of the infection. It also depends on the patient's kidney function. More detailed information can be obtained from a doctor or pharmacist.
The ready-to-use suspension should be administered undiluted before or during a meal.
The usual dose is
Adults
400 mg (= 20 ml of the ready-to-use suspension) once a day, in a single dose, or 200 mg (= 10 ml) twice a day, every 12 hours.
Elderly
In elderly patients, there is no need to modify the dose if kidney function is normal.
Adolescents over 12 years old
Adolescents over 12 years old should be given the same dose as adults.
Children under 12 years old
Children under 12 years old should be given 8 mg of cefixime per kilogram of body weight per day, in a single dose or in two divided doses every 12 hours.
Dosing recommendations are presented in the following table:
| Body weight | Daily dose [ml] Once daily | Daily dose [ml] Twice daily | Daily dose [mg] |
| 6.0-9 kg (infants over 6 months) | 1 × 2.5 ml | 2 × 1.25 ml | 50 mg |
| 10.0 kg | 4 ml | 2 × 2 ml | 80 mg |
| 12.5 kg | 5 ml | 2 × 2.5 ml | 100 mg |
| 15.0 kg | 6 ml | 2 × 3 ml | 120 mg |
| 17.5 kg | 7 ml | 2 × 3.5 ml | 140 mg |
| 20.0 kg | 8 ml | 2 × 4 ml | 160 mg |
| 22.5 kg | 9 ml | 2 × 4.5 ml | 180 mg |
| 25.0 kg | 10 ml | 2 × 5 ml | 200 mg |
| 27.5 kg | 11 ml | 2 × 5.5 ml | 220 mg |
| 30.0 kg | 12 ml | 2 × 6 ml | 240 mg |
| 37.5 kg | 15 ml | 2 × 7.5 ml | 300 mg |
| >37.5 kg | 20 ml | 2 × 10 ml | 400 mg |
For adolescents and adult patients without swallowing disorders, cefixime is recommended in tablet form.
Kidney function disorders
Cefixime can be administered to patients with kidney function disorders. In patients with a creatinine clearance of 20 ml/min or higher, the standard dose and dosing schedule can be used.
In patients with a creatinine clearance below 20 ml/min/1.73 m², the dose should not exceed 200 mg once a day.
Children under 12 years old with a creatinine clearance below 20 ml/min/1.73 m² should be given 4 mg of cefixime per kilogram of body weight only once a day.
Preparing the suspension
60 ml of oral suspension: to prepare the suspension, use the measuring cup provided in the carton. Add 40 ml of water divided into two portions and shake after each addition.
100 ml of oral suspension: to prepare the suspension, use the measuring cup provided in the carton. Add 66 ml of water divided into two portions and shake after each addition.
The ready-to-use suspension is a viscous liquid with a color ranging from almost white to light yellow.
Shake the bottle well before each use.
To measure the prescribed dose of the suspension, use the provided plastic oral syringe with a scale.
The plastic oral syringe is included in the packaging.
How to use the oral syringe
- 1. Before use, shake the bottle well and then remove the cap.
- 2. Remove the syringe cover and insert the syringe into the bottle.
- 3. Pull the plunger to fill the syringe to the prescribed dose marked on the plunger.
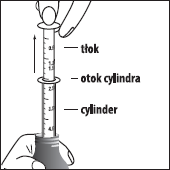
- 4. Remove the syringe from the bottle.
- 5. Place the tip of the syringe in the patient's mouth, against the inside of the cheek.
- 6. Slowly press the plunger to administer the medicine, without forcing it, to avoid choking. DO NOT administer the medicine in a strong stream.
- 7. Repeat steps 2-6 until the full dose is administered.
- 8. After administering the dose, replace the cap on the bottle. Disassemble the syringe and wash it thoroughly under running water. Allow the plunger and cylinder to air dry.
Duration of treatment
Treatment usually lasts 7 days. It can be extended to 14 days, depending on the severity of the infection.
Treatment of acute uncomplicated urinary tract infections in women lasts from 1 to 3 days.
Taking more Cetix than prescribed
If the patient accidentally takes more than the prescribed dose of Cetix, they should immediately consult a doctor or go to the nearest emergency department.
Missing a dose of Cetix
If the patient forgets to take a dose of Cetix, they should take it as soon as they remember. However, if there are less than 6 hours left before the next dose, they should skip the missed dose and return to their regular dosing schedule. Do not take a double dose to make up for the missed dose.
Stopping treatment with Cetix
It is essential to take this medicine until the end of the prescribed treatment cycle. Do not stop taking Cetix just because the patient feels better. Stopping treatment too early may lead to a recurrence of the infection. Consult a doctor if the patient still feels unwell or feels worse during treatment.
In case of any further doubts about taking the medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Cetix can cause side effects, although not everybody gets them.
The following side effects are important and require immediate action if they occur. If the patient experiences any of the following symptoms, they should stop taking Cetix and consult a doctor immediately.
Very rare(may occur in less than 1 in 10,000 patients):
- watery, severe diarrhea, which may also be bloody;
- sudden severe allergic reactions (anaphylactic shock), such as skin rash or hives, itching, swelling of the face, lips, tongue, or other parts of the body, chest tightness, wheezing, or collapse;
- severe skin disease with blistering of the skin, mouth, eyes, and genitals (Stevens-Johnson syndrome, toxic epidermal necrolysis) (see section 2. Important information before taking Cetix - Warnings and precautions).
Frequency not known(cannot be estimated from the available data)
- severe skin rashes, fever, swollen lymph nodes, and increased levels of white blood cells called eosinophils (DRESS) (see section 2. Important information before taking Cetix - Warnings and precautions).
The following side effects have also been reported.
Common(may occur in less than 1 in 10 patients):
- diarrhea.
Uncommon(may occur in less than 1 in 100 patients):
- headache;
- nausea;
- vomiting;
- abdominal pain;
- changes in blood test results that assess liver function;
- skin rash.
Rare(may occur in less than 1 in 1,000 patients):
- increased risk of infection caused by microorganisms that cefixime does not affect, e.g., thrush;
- increased levels of white blood cells called eosinophils;
- allergic reaction;
- loss of appetite;
- dizziness;
- bloating (gas);
- itching;
- inflammation of the mucous membrane of the mouth and/or other surfaces inside the body;
- fever;
- changes in blood test results that assess kidney function.
Very rare(may occur in less than 1 in 10,000 patients):
- decreased levels of various blood cells (symptoms may include fatigue, new infections, increased bruising or bleeding);
- allergic reaction characterized by skin rashes, fever, joint pain, and swollen lymph nodes;
- nervousness and increased activity;
- liver disorders, including jaundice (yellowing of the skin or whites of the eyes);
- kidney inflammation.
Frequency not known(cannot be estimated from the available data):
- increased platelet count (thrombocytosis);
- decreased levels of a type of white blood cell (neutropenia);
- indigestion;
- skin rash or skin changes with a red or pink ring and a white center, which may itch, flake, or be filled with fluid. The rash may appear especially on the palms and soles. These may be symptoms of a serious allergy to the medicine, called "erythema multiforme".
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist.
Side effects can be reported directly to the Department of Drug Adverse Reaction Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, PL-02 222 Warsaw, tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Cetix
Keep the medicine out of the sight and reach of children.
The granules should be stored at a temperature below 25°C.
The ready-to-use suspension can be stored for 14 days at a temperature below 25°C or in the refrigerator.
Do not use this medicine after the expiry date stated on the carton after "Expiry date". The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Cetix contains
- The active substance of Cetix is cefixime. Each 5 ml of the ready-to-use oral suspension contains 100 mg of cefixime in the form of cefixime trihydrate (111.9 mg).
- The other ingredients of the medicine are: sucrose, xanthan gum, sodium benzoate (E211), orange flavor (Flavour durarome orange) [flavoring substances, maltodextrin, sucrose, soy lecithin (E322), silicon dioxide (E551)].
What Cetix looks like and contents of the pack
Cetix, granules for oral suspension, is white to light yellow in color.
The granules for oral suspension are directly packaged in 150 ml brown glass bottles (type III) with an aluminum cap with a polyethylene seal.
The carton contains one (1) bottle, one plastic (polypropylene) measuring cup intended only for preparing the suspension, with a volume of 40 ml or 66 ml marked, one plastic oral syringe with a capacity of 5 ml and a scale from 0.5 ml to 5 ml with a graduation of 0.25 ml printed on the plunger, and a patient information leaflet. Each bottle contains 32 g of granules (1.2 g of cefixime) to prepare 60 ml of oral suspension or 53 g of granules (2 g of cefixime) to prepare 100 ml of oral suspension.
Marketing authorization holder
Aflofarm Farmacja Polska Sp. z o.o.
ul. Partyzancka 133/151
95-200 Pabianice
Tel. (42) 22-53-100
Importer
Alkaloid-INT d.o.o., Šlandrova ulica 4, 1231 Ljubljana–Črnuče, Slovenia
Tel.: +386 1 300 42 90
Fax: +386 1 300 42 91
e-mail: [email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Sorcelif 100 mg/5 ml Granulat zur Herstellung einer Suspension zum Einnehmen
Poland: CETIX
Portugal: Cefixima InnFarm
Czech Republic: Cefixime InnFarm 100 mg/5 ml granule pro perorální suspenzi
Slovakia: Cefixime InnFarm 100 mg/5 ml, granulát na perorálnu suspenziu
Romania: XIFIA 100 mg/5 ml granule pentru suspensie orală
Hungary: XIFIA 100 mg/5 ml granulátum belsőleges szuszpenzióhoz
Date of last revision of the leaflet:01.2024
Medical advice and education
Antibiotics are used to treat bacterial infections. They are ineffective in treating viral infections.
The antibiotic prescribed by the doctor is needed by the patient only to treat the current illness.
Some bacteria may survive or continue to multiply despite the use of antibiotics. This phenomenon is called resistance: treatment with some antibiotics becomes ineffective.
Improper use of antibiotics exacerbates resistance. You can even help bacteria develop resistance and delay treatment or reduce the effectiveness of the antibiotic if you do not follow the instructions for:
- 1. Dosage
- 2. Treatment schedule
- 3. Duration of treatment
As a result, to maintain the effectiveness of this medicine, follow the instructions below.
- 1. Take antibiotics only when prescribed by a doctor.
- 2. Follow the instructions carefully.
- 3. Do not take an antibiotic again without a doctor's prescription, even if the treatment is for a similar illness.
- 4. Never give your antibiotic to others; it may not be suitable for treating their illnesses.
- 5. After completing treatment, return any unused medicine to the pharmacy, so it can be disposed of properly.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAlkaloid - INT d.o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CetixDosage form: Powder, 1 gActive substance: cefotaximeManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Produkcyjny w DuchnicachPrescription not requiredDosage form: Powder, 2 gActive substance: cefotaximeManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Produkcyjny w DuchnicachPrescription not required
Alternatives to Cetix in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cetix in Spain
Alternative to Cetix in Ukraine
Online doctors for Cetix
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cetix – subject to medical assessment and local rules.














