

Calcium hloratum Vzf

Ask a doctor about a prescription for Calcium hloratum Vzf

How to use Calcium hloratum Vzf
Package Leaflet: Information for the Patient
CALCIUM CHLORATUM WZF, 67 mg/ml, Solution for Injection
Calcium chloride dihydrate
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Calcium chloratum WZF and what is it used for
- 2. Important information before using Calcium chloratum WZF
- 3. How to use Calcium chloratum WZF
- 4. Possible side effects
- 5. How to store Calcium chloratum WZF
- 6. Contents of the pack and other information
1. What is Calcium chloratum WZF and what is it used for
The medicine contains calcium chloride in the form of a solution for intravenous administration, which is used:
- in conditions of calcium deficiency requiring rapid supplementation;
- in poisoning: with calcium channel antagonists (if there are circulatory disorders: a drop in blood pressure, conduction disorders), magnesium sulfate, fluorides, and oxalates;
- in cardiopulmonary resuscitation;
- in significant hyperkalemia (elevated potassium levels in the blood) with changes in the ECG.
2. Important information before using Calcium chloratum WZF
When not to use Calcium chloratum WZF
- if the patient is allergic to calcium chloride or any of the other ingredients of this medicine (listed in section 6);
- if the patient has hypercalcemia (too high a level of calcium in the blood);
- if the patient has hypercalciuria (too high a level of calcium in the urine);
- if the patient has kidney stones;
- if the patient has acute kidney failure.
Warnings and precautions
Before starting treatment with Calcium chloratum WZF, discuss it with your doctor or pharmacist.
If the patient has been found to have a decreased calcium level in the serum resulting from kidney failure, respiratory acidosis, or respiratory failure, calcium chloride is not recommended due to its acidic properties.
Calcium salts should be administered with caution to patients with sarcoidosis.
Caution should be exercised when administering intravenous calcium preparations to patients with heart rhythm disorders, as calcium salts may increase the risk of arrhythmias.
The medicine should not be administered subcutaneously or intramuscularly, as it may cause tissue necrosis at the injection site.
The medicine should be administered slowly intravenously, into large veins or central veins, to avoid irritating the veins and prevent side effects. Particular caution should be exercised to avoid potential extravasation of the administered solution. Before each administration of calcium chloride, make sure the needle or catheter is in the vessel lumen.
In the event of extravasation of the solution and infiltration of surrounding tissues, administration of the medicine should be stopped immediately. Appropriate action should be taken to minimize the risk of damage to surrounding tissues. If possible, the extravasated solution should be carefully aspirated. Local injection of the extravasation area with a 1% procaine hydrochloride solution with the addition of hyaluronidase may reduce vasoconstriction and dilute the calcium solution extravasated into the surrounding tissues. Local application of a warm compress may also be helpful.
After injection of the medicine, a drop in blood pressure may occur.
Administration of the medicine too quickly and/or in too large doses, leading to high calcium levels in the blood reaching the heart, may cause a risk of loss of consciousness due to cardiac causes.
Calcium solutions and sodium bicarbonate solutions should not be administered simultaneously through the same vascular access.
Children
In infants, the medicine should not be injected into veins on the head. The medicine should not be given orally to infants, as it may cause severe irritation of the digestive tract.
Calcium chloratum WZF and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
- Intravenous administration of calcium salts to patients taking cardiac glycosides (e.g., digoxin) may cause dangerous heart rhythm disorders. If administration of the medicine is necessary in such a case, it should be administered slowly in small portions, preferably in a drip infusion in an intensive care unit.
- Thiazide diuretics (e.g., hydrochlorothiazide) used to treat hypertension may increase the risk of elevated calcium levels in the blood (hypercalcemia).
- Bisphosphonates (bisphosphonic acid derivatives) used to treat osteoporosis or Paget's disease may interact with calcium chloride, resulting in reduced absorption of bisphosphonates.
- Calcium salts reduce the absorption of tetracycline antibiotics.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
The medicine may be used in pregnant women if, in the doctor's opinion, the benefit to the mother outweighs the potential risk to the fetus.
Calcium chloride passes into breast milk in such small amounts that it does not affect the breastfed child.
Driving and using machines
The medicine does not affect driving or using machines.
Calcium chloratum WZF contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per 1 ml, i.e., the medicine is considered "sodium-free".
The medicine can be diluted in a 0.9% sodium chloride solution or a 5% glucose solution. The sodium content from the diluent should be taken into account when calculating the total sodium content in the prepared dilution of the medicine. For accurate information on the sodium content in the solution used to dilute the medicine, refer to the patient information leaflet of the diluent.
3. How to use Calcium chloratum WZF
This medicine should always be used as directed by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
Dosing is individual and depends on the degree of calcium deficiency. The medicine should be administered slowly, at a rate not exceeding 1 millimole of calcium ions per minute. If the patient experiences adverse symptoms, administration of the medicine should be stopped. Resumption of administration is possible after the symptoms have subsided. After injection of the medicine, the patient should remain in a lying position for a few minutes.
The medicine is intended for slow intravenous administration into large veins or central veins. The medicine should not be administered subcutaneously or intramuscularly, as it may cause tissue necrosis at the injection site.
The solution for administration should be at room temperature.
- Conditions of calcium deficiency requiring rapid supplementationAdults: 5 to 10 ml of the solution (2.3 mmol to 4.6 mmol of calcium ions) should be administered very slowly intravenously. The next dose can be administered after an interval of 1 to 3 days, depending on the patient's response and/or serum calcium levels. In cases of increased calcium excretion, it may be necessary to repeat the dose.
Children
0.2 ml/kg body weight/dose (0.092 mmol of calcium ions/kg body weight/dose) of the solution should be administered very slowly intravenously.
Maximum dose: 1 to 10 ml per day.
- Poisoning: with calcium antagonists (if there are circulatory disorders: a drop in blood pressure, conduction disorders), magnesium sulfate, fluorides, or oxalatesAdults: Initially, 5 ml of the solution (2.3 mmol of calcium ions) should be administered immediately after poisoning has been diagnosed. If necessary, the dose can be repeated.
- Cardiopulmonary resuscitationAdults: 5 to 10 ml of the solution (2.3 mmol to 4.6 mmol of calcium ions) should be administered very slowly intravenously.
- Significant hyperkalemia with changes in the ECGAdults: Calcium chloride should be administered only under ECG control.
Note:Calcium chloride should not be mixed with carbonates, phosphates, sulfates, acetates, or tetracyclines in solutions for parenteral administration.
The medicine can be diluted with a 0.9% sodium chloride solution or a 5% glucose solution. It is recommended to dilute the medicine in a ratio of at least 1:1 in the above-mentioned solutions. The solution obtained after dilution should be used immediately, due to the lack of preservatives in the medicine.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
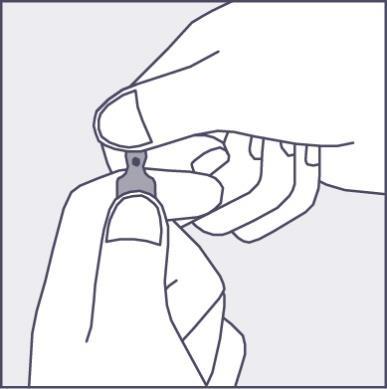
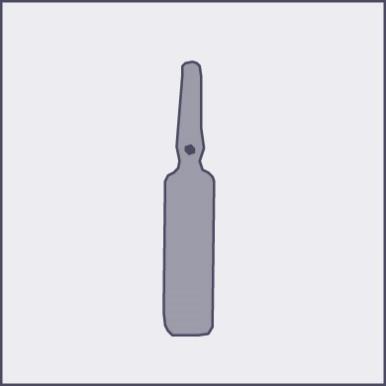
A colored dot (see Figure 1) has been placed on each ampoule as a mark indicating the location of the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
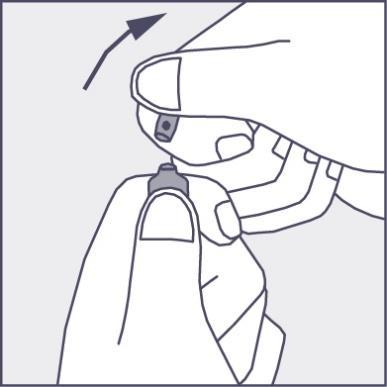
- Press in the direction of the arrow shown in Figure 3. The ampoules are intended for single use only and should be opened immediately before use. Any remaining contents of the unused product should be disposed of in accordance with applicable regulations.
Figure 1
Figure 2
Figure 3
Using a larger dose of Calcium chloratum WZF than recommended
Administration of large doses of calcium salts may lead to hypercalcemia; symptoms of hypercalcemia include: loss of appetite, nausea, vomiting, constipation, abdominal pain, muscle weakness, mental disorders, increased thirst, polyuria, kidney stones, and in severe cases, heart rhythm disorders and coma.
Treatment involves hydration of the patient, administration of loop diuretics, chelating agents, calcitonin, and corticosteroids. The serum calcium level should be frequently determined at equal time intervals to adjust the treatment to the patient's condition.
In case of any further doubts about the use of this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Injection of calcium salts may cause irritation. Intramuscular and subcutaneous administration of the medicine is associated with a particular risk of such an effect and is contraindicated.
During parenteral use of calcium salts, soft tissue calcification has been observed.
Administration of large doses of calcium salts may lead to hypercalcemia (see "Using a larger dose of Calcium chloratum WZF than recommended").
Too rapid intravenous injection of calcium salts may also lead to hypercalcemia and a feeling of chalky taste in the mouth, hot flashes, and peripheral vasodilation.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Calcium chloratum WZF
Do not freeze.
The chemical and physical stability of the diluted solution has been demonstrated for 24 hours at a temperature of 15°C-25°C.
From a microbiological point of view, the diluted product should be used immediately. If it is not used immediately, the user is responsible for the storage conditions and storage time.
The medicine should be stored out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
- The active substance of the medicine is calcium chloride dihydrate. Each 1 ml of the solution contains 67 mg of calcium chloride dihydrate, which corresponds to 0.46 mmol (18.3 mg) of calcium ions.
- The other ingredients are: sodium hydroxide 10% or hydrochloric acid 10% (to adjust the pH), water for injections.
What Calcium chloratum WZF looks like and contents of the pack
10 ampoules of 10 ml each
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Calcium hloratum VzfDosage form: Syrup, 116 mg Ca 2+/5 mlActive substance: calcium (different salts in combination)Manufacturer: Aflofarm Farmacja Polska Sp. z o.o.Prescription not requiredDosage form: Syrup, 116 mg calcium ions/5 mlActive substance: calcium (different salts in combination)Manufacturer: Aflofarm Farmacja Polska Sp. z o.o.Prescription not requiredDosage form: Tablets, 45 mg Ca2+Active substance: calcium gluconatePrescription not required
Alternatives to Calcium hloratum Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Calcium hloratum Vzf in Spain
Online doctors for Calcium hloratum Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Calcium hloratum Vzf – subject to medical assessment and local rules.














