Budezonid Lek-am

Ask a doctor about a prescription for Budezonid Lek-am

How to use Budezonid Lek-am
Leaflet attached to the packaging: patient information
Budezonid LEK-AM, 200 micrograms/inhalation dose,
inhalation powder in hard capsules
Budezonid LEK-AM, 400 micrograms/inhalation dose, inhalation powder in hard capsules
Budesonide
It is essential to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- The leaflet should be kept, so that it can be re-read if necessary.
- In case of any doubts, the patient should consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Budezonid LEK-AM and what is it used for
- 2. Important information before using Budezonid LEK-AM
- 3. How to use Budezonid LEK-AM
- 4. Possible side effects
- 5. How to store Budezonid LEK-AM
- 6. Contents of the packaging and other information
1. What is Budezonid LEK-AM and what is it used for
Budesonide, the active substance of Budezonid LEK-AM, belongs to a group of medicines called corticosteroids and is used to treat asthma and chronic obstructive pulmonary disease (COPD).
Budezonid LEK-AM is used to reduce inflammatory changes in the lungs. Asthma is caused by inflammation of the airways. Regular use of Budezonid LEK-AM helps prevent asthma attacks and makes breathing easier in chronic obstructive pulmonary disease.
The patient should not stop using Budezonid LEK-AM regularly, even after the symptoms of the disease have disappeared.
In case of any doubts about the way the medicine works and the reasons for its recommendation, the patient should consult a doctor.
2. Important information before using Budezonid LEK-AM
When not to use Budezonid LEK-AM:
- if the patient is allergic to budesonide or any other component of this medicine (listed in section 6). If the patient thinks they may be allergic, they should contact their doctor.
- if the patient has been diagnosed with pulmonary tuberculosis (current or past). If any of the above situations apply to the patient, they should not use Budezonid LEK-AM and should inform their doctor as soon as possible.
Warnings and precautions
Before starting to use Budezonid LEK-AM, the patient should discuss it with their doctor if:
- the patient is using another corticosteroid medicine at the same time,
- the patient has difficulty breathing related to diseases of the airways other than asthma or COPD.
The patient should immediately inform their doctor if they experience any of the following symptoms:
- respiratory tract infection while using Budezonid LEK-AM (possible symptoms: severe cough, fever, discharge in the airways),
- difficulty breathing with wheezing or cough after using Budezonid LEK-AM,
- rash, itching, hives, difficulty breathing or swallowing, dizziness, or swelling of the face or throat while using Budezonid LEK-AM,
- weight change, weakness, abdominal obesity, nausea, persistent diarrhea while using Budezonid LEK-AM,
- blurred vision or other vision disturbances while using Budezonid LEK-AM,
- sleep problems, depression, or feelings of anxiety, restlessness, nervousness, excessive stimulation, or irritability during treatment with Budezonid LEK-AM.
Other special warnings
- The patient should not swallow the Budezonid LEK-AM capsules - they should only be used for inhalation using the inhaler provided with the packaging.
- The Budezonid LEK-AM capsules should only be used with the inhaler provided with the packaging. The patient should not use another inhaler.
- If the symptoms worsen, such as wheezing or shortness of breath, the patient should inform their doctor.
- The patient should not useBudezonid LEK-AM to treat acute shortness of breath attacks. In these cases, other medicines are used. If the patient has not been prescribed another medicine for this purpose, they should discuss it with their doctor.
- The patient should not suddenly stop using oral anti-inflammatory medicines, such as oral steroids. In patients who have been treated with oral anti-inflammatory medicines for a long time, the doctor will gradually reduce the dose of these medicines while starting to use Budezonid LEK-AM. The doctor may recommend that the patient carry a warning card, as additional anti-inflammatory treatment may be necessary in case of an accident, surgery, or severe infection.
- The patient should rinse their mouth with water after each use of Budezonid LEK-AM to reduce the risk of fungal infections of the mouth. The patient should not swallow the water.
- To alleviate asthma symptoms, the patient should always carry a short-acting bronchodilator (such as albuterol or salbutamol).
- Periodically, the doctor may perform a test of adrenal function.
- It is recommended that patients always carry a card indicating that they have asthma.
- In case of worsening asthma symptoms, the doctor may recommend increasing the dose of Budezonid LEK-AM or using oral corticosteroids and/or an antibiotic in case of infection.
- In stressful situations, such as injury or surgery, the doctor may recommend additional corticosteroids.
- Replacing systemic corticosteroids with inhaled corticosteroids may reveal the existence of allergic reactions, such as allergic rhinitis or eczema, which were previously suppressed by oral corticosteroids. Patients may experience fatigue, muscle or joint pain, nausea, or vomiting. Allergic reactions can be treated with antihistamines or topical corticosteroids.
Allergic reactions may be treated with antihistamines or topical corticosteroids.
- If the patient has been diagnosed with liver disease, they should inform their doctor before using Budezonid LEK-AM. The doctor will recommend an appropriate dose of the medicine.
Children and adolescents
Budezonid LEK-AM should not be used in children under 6 years of age.
If a child uses a high dose of inhaled steroids for a long time, the doctor will regularly monitor the child's growth.
Budezonid LEK-AM and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. This is especially important for:
- certain medicines used to treat infections (such as itraconazole, ketoconazole, clarithromycin, rifampicin),
- certain medicines used to treat HIV infections (such as ritonavir, nelfinavir, atazanavir),
- certain medicines used to treat heart rhythm disorders (such as amiodarone). If the patient is taking any of these medicines, a dose change or other precautions may be necessary.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine may be used during pregnancy only if the doctor believes that the benefits to the mother outweigh the potential risks to the fetus. The doctor will discuss the risks of using the medicine during pregnancy with the patient.
It is not recommended to use Budezonid LEK-AM during breastfeeding. If the patient is breastfeeding, they should tell their doctor before using this medicine. The doctor will discuss the risks of using the medicine during breastfeeding with the patient.
Driving and using machines
It is unlikely that Budezonid LEK-AM will affect the patient's ability to drive or use machines.
Budezonid LEK-AM contains lactose (milk sugar)
If the patient has been diagnosed with intolerance to certain sugars, such as lactose, they should contact their doctor before using Budezonid LEK-AM.
3. How to use Budezonid LEK-AM
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor. The patient should not exceed the recommended dose.
Recommended dose
Asthma
Adults: The recommended dose is 200 to 400 micrograms of budesonide once or twice a day.
In case of worsening asthma symptoms, when switching from oral corticosteroids to budesonide inhalation or reducing the dose of oral corticosteroids, the dose of budesonide may be increased to 1600 micrograms per day, divided into 2 to 4 inhalations.
Children over 6 years old:The recommended dose is 200 micrograms of budesonide once or twice a day. If necessary, the doctor may increase the dose of budesonide to 800 micrograms per day. Children should use Budezonid LEK-AM under adult supervision.
Chronic obstructive pulmonary disease
Adults: The recommended dose is 200 to 400 micrograms of budesonide twice a day. If necessary, the doctor may increase the dose to 1600 micrograms per day.
In case of doubts about using the medicine, the patient should consult their doctor or pharmacist.
Depending on the patient's response to the treatment, the doctor may recommend a higher or lower dose of the medicine.
When to use Budezonid LEK-AM
Budezonid LEK-AM is best used at the same time every day.
If the patient feels that the effect of Budezonid LEK-AM is too strong or too weak, they should consult their doctor.
How to use Budezonid LEK-AM
The patient should follow the instructions below.
The Budezonid LEK-AM capsules should only be used for inhalation using the inhalerprovided with the packaging.
The patient should not swallow the capsules. The powder in the capsule is for inhalation only.
If the patient experiences difficulty breathing or wheezing after using Budezonid LEK-AM, they should stop using the medicine and consult their doctor immediately.
Before starting to use the medicine, the patient should read the "Inhaler instruction manual".
Inhaler instruction manual
The inhaler is designed to deliver the powder from the number of capsules in the packaging. The patient should not use the same inhaler for another packaging of the medicine.
- 1. Remove the inhaler cap.
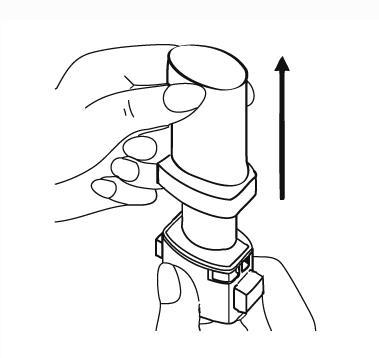
- 2. Open the inhaler. To do this, hold the base of the inhaler firmly and turn the mouthpiece in the direction indicated by the arrow.
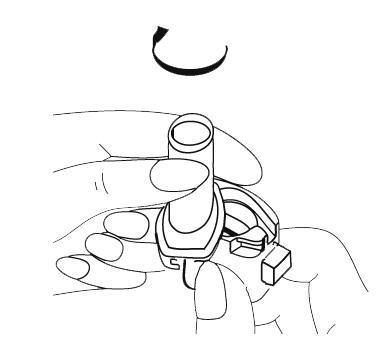
- 3. Remove 1 capsule from the blister pack and place it in the chamber of the inhaler base. The capsule should be removed from the foil blister pack immediately before use. IMPORTANT: do not place the capsule in the mouthpiece.
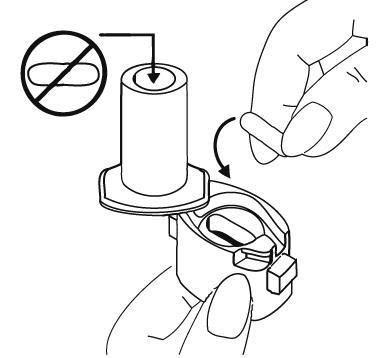
- 4. Close the inhaler chamber by turning the mouthpiece in the direction indicated by the arrow (until a click is heard).
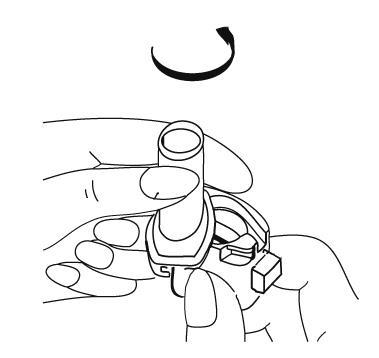
- 5. Holding the inhaler upright with the mouthpiece pointing upwards, press the inhaler buttons once, until they stop.
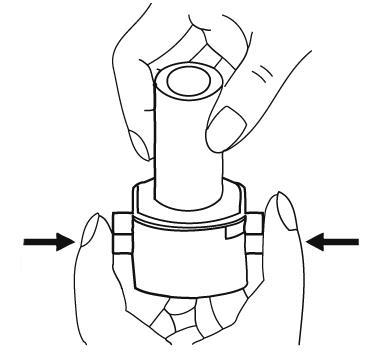
- 6. Release the inhaler buttons. NOTE:at this point, the capsule may break, and small pieces of the capsule shell may enter the mouth or throat. Swallowing a piece of the shell is not harmful. The likelihood of this happening is minimal if the capsule is not pierced more than once, the storage conditions are maintained, and the capsule is removed from the blister pack immediately before use (see point 3).
- 7. Exhale slowly and deeply. Do not exhale into the inhaler.
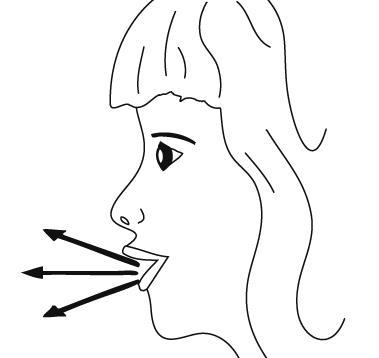
- 8. Tilt the head slightly backwards, put the inhaler mouthpiece in the mouth, and seal it with the lips.
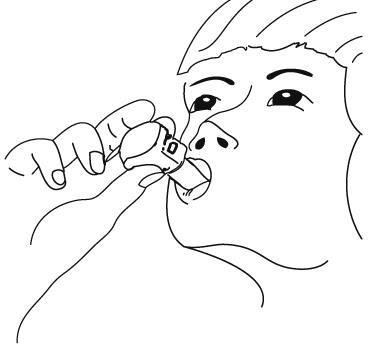
- 9. Take a quick, deep, and even breath. During inhalation, a characteristic sound should be heard. If this sound does not appear, it may mean that the capsule is stuck in the inhaler chamber. In this case, the patient should open the inhaler and remove the capsule from the chamber by lifting it. The patient should nottry to remove the capsule by pressing the buttons multiple times. Then, the patient should repeat the steps described in point 9.
- 10. After inhalation, the patient should hold their breath for as long as possible without feeling uncomfortable. Then, the patient should remove the inhaler from their mouth and exhale through their nose.
- 11. Open the inhaler and check if there is still powder in the capsule. If powder remains in the capsule, the patient should repeat the procedure from point 7 to point 10.
- 12. After use, open the inhaler (see point 2 of this instruction), remove the empty capsule. To remove any remaining powder, the patient should wipe the mouthpiece and the chamber of the inhaler (where the capsule is placed) with a drycloth. The patient can also use a soft brush for this purpose. The patient should not use water to clean the inhaler.
- 13. Close the inhaler chamber and put the cap back on.
How long to use Budezonid LEK-AM
It is essential to use Budezonid LEK-AM regularly as directed by the doctor. The patient should use Budezonid LEK-AM even when asthma symptoms do not occur, as it helps prevent asthma attacks. If the patient has doubts about how long to use Budezonid LEK-AM, they should consult their doctor.
Using a higher dose of Budezonid LEK-AM than recommended
If the patient has taken a higher dose of the medicine than recommended, or if someone else has taken the medicine by mistake, they should immediately consult their doctor or go to the hospital. The patient should take the packaging of the medicine with them. Appropriate treatment may be necessary.
It is essential to use the doses of the medicine as recommended by the doctor. The patient should not increase or decrease the dose without consulting their doctor.
Missing a dose of Budezonid LEK-AM
If the patient misses a dose of the medicine, they should take the next dose at the usual time. The patient should not take a double dose to make up for the missed dose.
Stopping the use of Budezonid LEK-AM
Stopping the use of Budezonid LEK-AM may cause worsening of asthma symptoms. The patient should not suddenly stop using Budezonid LEK-AM unless their doctor recommends it.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Budezonid LEK-AM can cause side effects, although not everybody gets them.
Some side effects can be serious.
Side effects that occur in 1 to 10 out of 10,000 patients
- Difficulty breathing with wheezing, cough.
- Severe skin allergic reactions with rash, itching, hives, difficulty breathing or swallowing, dizziness, and/or swelling of the face and throat.
- Extreme weakness, weight loss, nausea, persistent diarrhea; these may be symptoms of adrenal insufficiency.
- Weight gain, moon face, weakness, and/or abdominal obesity; these may be symptoms of a hormonal disorder, known as Cushing's syndrome.
- Visual disturbances: blurred vision (cataract) or increased pressure in the eye (glaucoma). If the patient experiences any of these symptoms, they should immediately contact their doctor.
Common side effects
Side effects that occur in 1 to 10 out of 100 patients
- Pneumonia (lung infection) in patients with COPD.
The patient should tell their doctor if they experience any of the following symptoms, which may be signs of a lung infection:
- fever or chills
- increased production of mucus, change in the color of mucus
- worsening cough or increased difficulty breathing.
Uncommon side effects
Side effects that occur in 1 to 10 out of 1,000 patients
- Blurred vision.
Rare side effects
Side effects that occur in 1 to 10 out of 10,000 patients
- Delayed growth in children and adolescents.
- Weakening of bone structure.
- Oral and throat infections (such as thrush).
- Temper tantrums or other behavioral problems (including depression) in children.
- Hoarseness.
- Pain or irritation of the throat.
If the patient experiences any of these side effects, they should inform their doctor.
Other side effects with unknown frequency
- Sleep problems, depression, or feelings of anxiety, restlessness, nervousness, excessive stimulation, or irritability. These side effects are more likely to occur in children.
- Contact dermatitis, which can occur as a result of contact between the skin and certain external substances (allergens or irritants) .If the patient experiences any of these side effects, they should inform their doctor.
Other side effects, reported in the literature, in patients taking similar medicines (containing the same active substance as Budezonid LEK-AM) for a long time (3 years) to treat COPD:
- Bruises. If this side effect occurs to a significant extent, the patient should immediately contact their doctor.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych,
Al. Jerozolimskie 181C
02-222 Warszawa,
phone: +48 22 49 21 301
fax: +48 22 49 21 309
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, it is possible to gather more information on the safety of the medicine.
5. How to store Budezonid LEK-AM
Store in the original packaging.
The medicine should be stored in a place that is out of sight and reach of children.
The patient should not use this medicine after the expiry date stated on the packaging after "Expiry date". The expiry date refers to the last day of the month.
The patient should not use this medicine if they notice that the packaging is damaged.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Budezonid LEK-AM contains
- The active substance of Budezonid LEK-AM is budesonide. One capsule (i.e., one inhalation dose) of Budezonid LEK-AM contains 200 or 400 micrograms of budesonide.
- Other ingredients of the medicine are: lactose monohydrate 230, lactose monohydrate 251, and capsule shell: hydroxypropyl methylcellulose, purified water.
What Budezonid LEK-AM looks like and contents of the packaging
Budezonid LEK-AM is a powder for inhalation in transparent, colorless capsules. The powder in the capsule is intended for inhalation into the lungs using the capsule inhaler provided with the packaging.
The packaging contains 30, 60, 90, or 120 capsules in blisters, as well as an inhaler.
Marketing authorization holder and manufacturer
Przedsiębiorstwo Farmaceutyczne LEK-AM Sp. z o.o.
ul. Ostrzykowizna 14A
05-170 Zakroczym
Polska
phone: +48 22 785 27 60
fax: +48 22 785 27 60 ext. 106
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterPrzedsiębiorstwo Farmaceutyczne LEK-AM Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Budezonid Lek-amDosage form: Suspension, 0.125 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.25 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.5 mg/mlActive substance: budesonidePrescription required
Alternatives to Budezonid Lek-am in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Budezonid Lek-am in Ukraine
Alternative to Budezonid Lek-am in Spain
Online doctors for Budezonid Lek-am
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Budezonid Lek-am – subject to medical assessment and local rules.