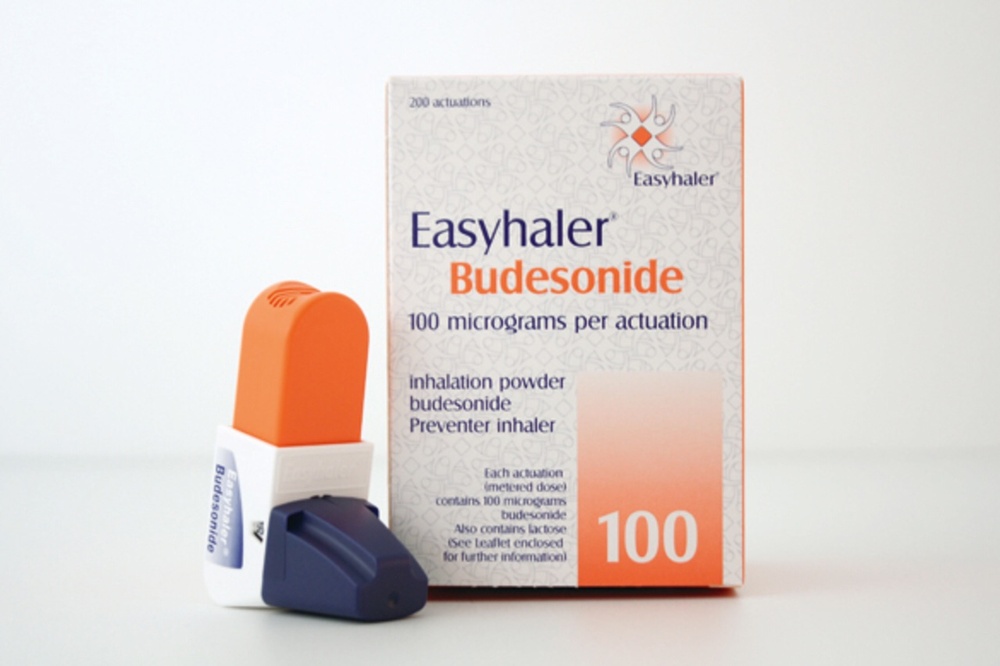
Budesonide Easihaler

Ask a doctor about a prescription for Budesonide Easihaler

How to use Budesonide Easihaler
1. What is Budesonide Easyhaler and what is it used for
The active substance of Budesonide Easyhaler is budesonide. Budesonide is in the form of a powder in an inhaler called Easyhaler. The powder is inhaled through the mouthpiece of the inhaler into the lungs. Budesonide works by reducing and preventing swelling and inflammation in the lungs.
Budesonide Easyhaler belongs to a group of medicines used preventively.It is used to prevent asthma symptoms. It belongs to a group of medicines called corticosteroids.
Budesonide will notstop an asthma attack that has already started. That's why you should always carry a fast-acting rescuemedicine for widening the airways (a beta-agonist) with you.
The noticeable effect of budesonide may occur only after a few days of treatment, and the maximum effect occurs after a few weeks. Budesonide Easyhaler should be used regularly to prevent inflammation associated with asthma. This medicine should be taken systematically, according to the recommendations, even if the patient does not experience any symptoms.
2. Important information before using Budesonide Easyhaler
When not to use Budesonide Easyhaler
If the patient is allergic to:
- budesonide,
- another component of this medicine (listed in section 6), which is lactose (containing small amounts of milk protein).
In such a case, the patient should contact their doctor, who will change the medicine to another one.
Warnings and precautions
The patient should inform their doctor beforeusing Budesonide Easyhaler if they have:
- pulmonary tuberculosis,
- any untreated bacterial, viral, or fungal infection of the mouth, respiratory tract, including the lungs,
- severe liver disease.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
After finishing inhalation, the patient should rinse their mouth to prevent fungal infection of the mouth.
Budesonide Easyhaler is not recommended for use in children under 6 years of age.
Budesonide Easyhaler and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription.
Some medicines may enhance the effect of Budesonide Easyhaler, and the doctor may want to closely monitor the patient's condition when taking such medicines (including some HIV medicines: ritonavir, cobicistat).
The patient should tell their doctor if they are using:
- nose sprays containing corticosteroids,
- corticosteroid tablets,
- antifungal medicines containing itraconazole or ketoconazole.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine. Both for the fetus and the mother, it is essential to ensure adequate treatment of asthma during pregnancy. As with other medicines used during pregnancy, the benefits and risks of using budesonide for the mother should be weighed against the potential risk to the fetus. The patient should use the smallest effective dose of budesonide to ensure adequate control of asthma.
Driving and using machines
Budesonide Easyhaler has not been shown to affect the ability to drive or use machines.
The Budesonide Easyhaler inhalation powder contains a small amount of lactose, which is unlikely to cause problems even if the patient has lactose intolerance. If the patient has intolerance to some sugars, they should consult their doctor before using this medicine.
Lactose contains small amounts of milk protein, which may cause an allergic reaction.
3. How to use Budesonide Easyhaler
This medicine should always be used according to the doctor's recommendations. If the patient has any doubts, they should consult their doctor or pharmacist.
The doctor has prescribed the appropriate strength and dose of the medicine for the patient. The patient should follow the doctor's instructions carefully.
Replacing corticosteroid tablets with Budesonide Easyhaler:
The patient should inform their doctor if they have been using corticosteroid tablets (e.g., cortisone tablets) to treat asthma. The doctor may recommend gradually reducing the number of tablets and eventually stopping treatment over several weeks. If the patient feels unwell during this period of gradual withdrawal, they should contact their doctor, but they should not stop using Budesonide Easyhaler.
Use in adults (including the elderly) and adolescents (12-17 years):
- 1-2 doses inhaled in the morning and evening or once a day in the evening.
Use in children (6-11 years):
- 1-2 doses inhaled in the morning and evening or once a day in the evening.
During treatment, the doctor may modify the dose of the medicine from time to time to establish the smallesteffective dose necessary to keep the patient's asthma under control (maintenance dose).
If Budesonide Easyhaler is to be used by a child, it is essential to ensure that they can use the medicine correctly.
In addition to the preventive medicine - Budesonide Easyhaler, the patient will also need a rescue medicine for widening the airways:
Budesonide will notstop an asthma attack that has already started. That's why the patient should always carry a fast-acting rescuemedicine for widening the airways (a beta-agonist) in case of sudden asthma symptoms.
If the patient is using a rescue inhaler (beta-agonist) regularly, they should use it before administering Budesonide Easyhaler (the preventive medicine).
Worsening of asthma symptoms during treatment:
The patient should contact their doctor as soon as possible if they experience:
- worsening wheezing or chest tightness during treatment
- need to use the rescue medicine more frequently than before
- the rescue medicine is not as effective as before. In this case, asthma may be worsening, and additional treatment may be necessary.
Using a higher dose of Budesonide Easyhaler than recommended
If a higher dose or more doses than recommended are used, the patient should tell their doctor as soon as possible. Unless the doctor recommends otherwise, the patient should continue using Budesonide Easyhaler.
It is essential to take the dose of the medicine as instructed in the leaflet or according to the doctor's recommendations. The patient should not increase or decrease the dose of the medicine without consulting their doctor.
Missing a dose of Budesonide Easyhaler
If a dose is missed, the patient should take it as soon as possible or take the next dose according to the dosing schedule. It is best to take the medicine at the same time every day.
The patient should not take a double dose to make up for a missed dose.
Stopping use of Budesonide Easyhaler
The patient should not stop using Budesonide Easyhaler without consulting their doctor. If the patient suddenly stops using this medicine, their asthma symptoms may worsen.
If the patient has any further doubts about using this medicine, they should consult their doctor, pharmacist, or nurse.
Instructions for using the Easyhaler inhaler are at the end of the leaflet.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common( may affect up to 1 in 10 people)
Irritation of the throat, difficulty swallowing, cough, and fungal infections (thrush) in the mouth or throat. If the patient experiences any of these side effects, they should not stop using Budesonide Easyhaler. They should consult their doctor.
The risk of the above side effects can be reduced by rinsing the mouth and throat with water or brushing the teeth after each dose of the medicine. The patient should not swallow the water after rinsing; they should spit it out.
Uncommon( may affect up to 1 in 100 people)
Anxiety, depression, cataract, blurred vision, muscle cramps, and tremors.
Rare( may affect up to 1 in 1,000 people)
Too high or too low levels of cortisol in the blood. Weakness of the adrenal glands (glands located near the kidneys). Skin rash, itching, bruising, hoarseness, and restlessness. Delayed growth in children.
Changes in behavior, particularly in children.
Rare, severe allergic reactions:
If the patient experiences itching, rash, redness of the skin, swelling of the eyelids, lips, or throat, wheezing, low blood pressure, or fainting soon after using the medicine, they should:
- stop using Budesonide Easyhaler
- contact their doctor immediately.
Shortness of breath immediately after using the medicine:
Rarely, inhaled medicines can cause worsening of wheezing and shortness of breath (bronchospasm) immediately after using the medicine. In this case, the patient should:
- stop using Budesonide Easyhaler
- use a fast-acting medicine for widening the airways
- contact their doctor immediately.
Very rare( may affect up to 1 in 10,000 people)
Glaucoma. Decreased bone density (weakening of bones).
Unknown( frequency cannot be estimated from the available data) :
Sleep problems, aggression, psychomotor hyperactivity (difficulty staying still), and irritability. The occurrence of these side effects is much more likely in children.
If the patient thinks they have experienced any of these rare side effects or is concerned that they may occur, they should talk to their doctor.
Reporting side effects
If the patient experiences any side effects, including any not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Medicinal Product Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw, phone: 22 49-21-301, fax: 22 49-21-309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Budesonide Easyhaler
The medicine should be stored out of sight and reach of children.
Before first use: store in a closed foil bag.
After opening the foil bag: store at a temperature below 30°C, protect from moisture. It is recommended to store the Easyhaler inhaler in its protective packaging.
The Budesonide Easyhaler inhaler can be used for a maximum of 6 months after opening the foil bag.
To remember the date of opening the bag, the patient should write it down. __________
If the Budesonide Easyhaler gets wet, it should be replaced with a new one.
Do not use this medicine after the expiry date stated on the label and carton after: "EXP".
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer use. This will help protect the environment.
6. Contents of the packaging and other information
What Budesonide Easyhaler contains
- The active substance is budesonide.
- The other ingredient is lactose monohydrate (which contains milk proteins).
What Budesonide Easyhaler looks like and contents of the pack
White or almost white powder.
Budesonide Easyhaler 100 micrograms/dose inhalation powder
- 200 doses + protective packaging for the inhaler
- 200 doses
- 2 x 200 doses
- 600 doses (3 x 200 doses)
Budesonide Easyhaler 200 micrograms/dose inhalation powder
- 120 doses
- 200 doses + protective packaging for the inhaler
- 200 doses
- 2 x 200 doses
- 600 doses (3 x 200 doses)
Budesonide Easyhaler 400 micrograms/dose inhalation powder
- 100 doses + protective packaging for the inhaler
- 100 doses
- 2 x 100 doses
Not all pack sizes may be marketed.
Marketing authorization holder
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
Manufacturer
Orion Corporation, Orion Pharma
Orionintie 1
02200 Espoo
Finland
To get more detailed information about this medicine, the patient should contact the local representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o. o.
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Finland, Hungary, Germany
Budesonid Easyhaler
Spain
Budesonida Easyhaler
Belgium, Poland
Budesonide Easyhaler
Slovenia
Budesonid Orion Easyhaler
Netherlands
Budesonide Orion Easyhaler
United Kingdom
Easyhaler Budesonide
Norway, Sweden, Denmark, Czech Republic, Slovakia, Austria, Estonia, Lithuania, Latvia, Malta
Giona Easyhaler
Date of last revision of the leaflet:08.06.2021
Detailed and up-to-date information about the use of this medicine is available after scanning the QR code (located on both the outer packaging and the inhaler label) using a smartphone.
The same information is available on the website: www.oeh.fi/bupl
QR code for the website www.oeh.fi/bupl
How to use the Easyhaler inhaler
Information about the Easyhaler inhaler
The Budesonide Easyhaler inhaler may differ from inhalers the patient has used before. Therefore, it is crucial to use it correctly, as incorrect use may result in the patient not receiving the correct amount of medicine. This may lead to worsening of the patient's condition or inadequate treatment of asthma.
The doctor, nurse, or pharmacist will show the patient how to use the inhaler correctly.
The patient should make sure they understand how to use the inhaler correctly. If they have any doubts, they should contact their doctor, nurse, or pharmacist. As with all inhalers, caregivers should ensure that children prescribed Budesonide Easyhaler use the correct inhalation technique, as described below. The patient can also use the video instructions at: www.oeh.fi/bupl
Using the Easyhaler inhaler for the first time
| The Easyhaler inhaler is supplied in a foil bag. Do not open the bag until you are ready to start using the medicine, as it helps keep the powder in the inhaler dry. If you are ready to start treatment, open the packaging and write down the opening date, e.g., in a calendar. The inhaler can be used for 6 months after removal from the foil bag. | 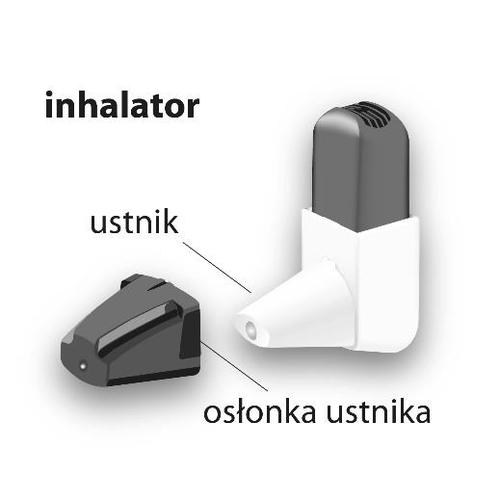 |
HOW TO USE THE INHALER
Step 1: SHAKING
| SHAKE 3-5 TIMES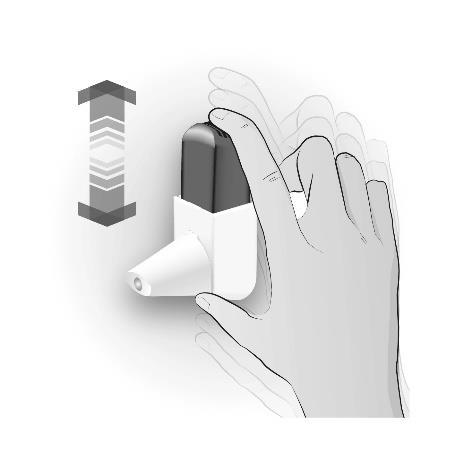 | Important information to remember
|
Step 2: CLICKING
| CLICK ONCE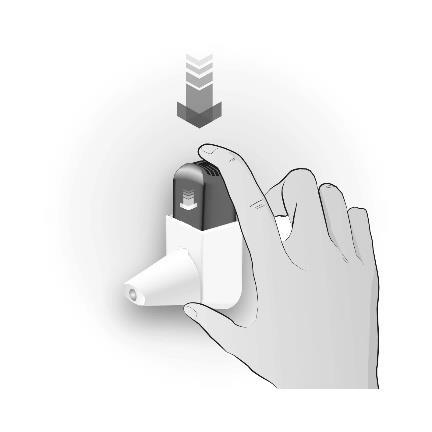 | Important information to remember
|
If additional inhalations are required, repeat the steps described in points 1-3 "Shaking-Clicking-Inhaling".
After using the inhaler:
- Put the mouthpiece cover back on to prevent accidental activation of the inhaler.
- Rinse your mouth and throat with water and spit out the water and (or) brush your teeth. This will reduce the risk of fungal infection (thrush) and hoarseness.
How to remove powder from the mouthpiece If the inhaler is accidentally clicked or clicked multiple times, or if you exhale into the inhaler, remove the powder from the mouthpiece:
|  |
Cleaning the Easyhaler inhaler
The inhaler should be kept dry and clean. If necessary, the mouthpiece of the inhaler can be cleaned with a dry cloth or tissue. Do not use water. The powder in the Easyhaler inhaler is sensitive to moisture.
Using the Easyhaler inhaler in the protective packaging The inhaler can be used in the protective packaging, which will increase the product's durability. Before putting the inhaler in the protective packaging, make sure the mouthpiece cover is covering the mouthpiece to prevent accidental activation of the inhaler. The inhaler can be used without removing it from the protective packaging. Use according to the instructions above,
| 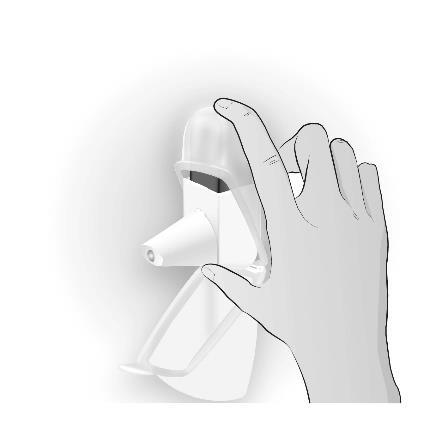 |
| Switching to a new Easyhaler inhaler The inhaler has a dose counter that shows the number of doses left. The counter turns every five activations. When the dose counter turns red, it means there are 20 doses left. If the patient does not have a new Easyhaler inhaler yet, they should contact their doctor to get a new prescription. When the dose counter shows 0, the patient should replace the Easyhaler inhaler. If the patient is using the protective packaging, they should keep it and put the new inhaler in it. | 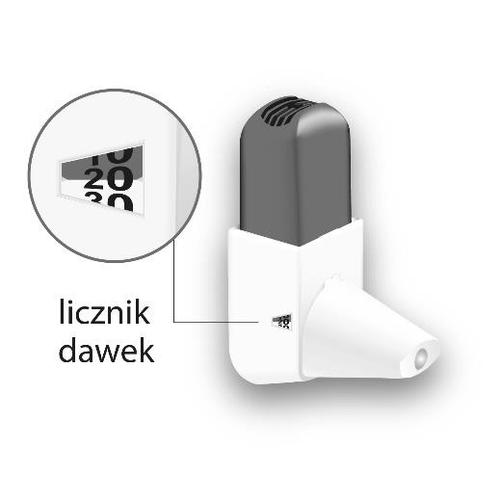 |
Remember
- 1. Shaking, 2. Clicking, 3. Inhaling.
- After taking a dose, rinse your mouth with water and spit it out and (or) brush your teeth.
- Do not let the inhaler get wet, and protect it from moisture.
If the patient has any further questions about using this medicine, they should ask their doctor or pharmacist.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOrion Corporation Orion Pharma
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Budesonide EasihalerDosage form: Suspension, 0.125 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.25 mg/mlActive substance: budesonidePrescription requiredDosage form: Suspension, 0.5 mg/mlActive substance: budesonidePrescription required
Alternatives to Budesonide Easihaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Budesonide Easihaler in Ukraine
Alternative to Budesonide Easihaler in Spain
Online doctors for Budesonide Easihaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Budesonide Easihaler – subject to medical assessment and local rules.