
Boostrix

Ask a doctor about a prescription for Boostrix

How to use Boostrix
Leaflet accompanying the package: information for the user
Boostrix, Suspension for injection in a pre-filled syringe
Pertussis, tetanus and diphtheria vaccine (acellular, composite), adsorbed, with reduced antigen content
Read the leaflet carefully before using the vaccine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Boostrix vaccine and what is it used for
- 2. Important information before using Boostrix vaccine
- 3. How to use Boostrix vaccine
- 4. Possible side effects
- 5. How to store Boostrix vaccine
- 6. Contents of the pack and other information
1. What is Boostrix vaccine and what is it used for
Boostrix is used for booster vaccination of children from 4 years of age, adolescents and adults to prevent three diseases: diphtheria, tetanus and pertussis (whooping cough). The vaccine causes the body to produce its own immunity (antibodies) against these diseases.
- Diphtheria:Diphtheria most often affects the respiratory tract and sometimes the skin. Usually, in the respiratory tract, there is inflammation and swelling, which can cause serious breathing difficulties and sometimes suffocation. Diphtheria bacteria also produce toxins that can cause nerve damage, heart disease, and even death.
- Tetanus:Tetanus bacteria enter the human body through a cut, scratch, or wound in the skin. Injuries that pose the greatest risk of tetanus infection include: burns, fractures, deep or dirty wounds, wounds contaminated with soil, dust, horse manure, or wood splinters. These bacteria produce toxins that can cause muscle stiffness, painful muscle spasms, convulsions, and even death. Muscle spasms can be so strong that they lead to spinal fractures.
- Pertussis (whooping cough):Pertussis is a highly contagious disease. The disease affects the respiratory tract, causing severe coughing attacks that can make normal breathing difficult. The cough in this disease is very characteristic - people with pertussis are said to "cough violently". The cough can last for 1-2 months or longer. Pertussis bacteria can also cause ear infections, bronchitis, which can last a long time, pneumonia, convulsions, brain damage, and even death.
None of the vaccine components can cause diphtheria, tetanus, or pertussis. Using the Boostrix vaccine during pregnancy helps protect the baby from pertussis during the first few months of the baby's life, before the baby receives the primary vaccination.
2. Important information before using Boostrix vaccine
When not to use Boostrix vaccine:
- if you have ever had an allergic reaction (hypersensitivity) to Boostrix or any component of this vaccine (listed in section 6) or to formaldehyde. Allergy symptoms include: itchy skin rash, difficulty breathing, swelling of the face or tongue.
- if you have ever had an allergic reaction to any pertussis, diphtheria or tetanus vaccine.
- if you have had any nervous system disorders within 7 days of previous administration of a pertussis vaccine.
- if you have a high fever (above 38.0 ° C). A mild infection, such as a cold, should not be a contraindication to vaccination, but you should tell your doctor first.
- if you have had a temporary decrease in platelet count (which increases the risk of bleeding or bruising) or brain and nerve disorders after previous vaccinations against diphtheria and/or tetanus.
Warnings and precautions
Before receiving the Boostrix vaccine, tell your doctor or pharmacist.
- if you have had any health problems after previous vaccinations with Boostrix or other pertussis vaccines, especially:
- High fever (above 40 ° C) within 48 hours of vaccination
- Shock or shock-like state within 48 hours of vaccination
- Persistent crying lasting at least three hours within 48 hours of vaccination
- Seizures or febrile seizures within 3 days of vaccination
- if your child has an undiagnosed or progressive brain disease or uncontrolled epilepsy. The vaccine should be given after the disease has been controlled
- if you have bleeding or bruising
- if you have a tendency to febrile seizures or if such cases have occurred in your family
- if you have prolonged immune system disorders (including HIV infection). In such cases, the Boostrix vaccine may be given, but vaccinated patients may not receive as good protection against infections as people with a normal immune system.
Fainting (syncope) can occur after or even before receiving any injectable vaccine (especially in adolescents). Therefore, you should inform your doctor or nurse if you have ever fainted while receiving an injection. As with any vaccine, it is possible that not all vaccinated individuals will develop a protective immune response.
Boostrix vaccine and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take and any vaccines you have recently received. The Boostrix vaccine can be given at the same time as some other vaccines. Each vaccine will be given in a different location. The Boostrix vaccine may not work properly if given at the same time as medicines that reduce the ability of the immune system to fight infections.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or plan to have a child, ask your doctor or pharmacist for advice before using this vaccine. It is not known whether Boostrix passes into breast milk. Your doctor will discuss with you the potential risks and benefits of using the Boostrix vaccine during breastfeeding.
Driving and using machines
It is unlikely that the Boostrix vaccine will affect your ability to drive or use machines.
Boostrix vaccine contains sodium
This vaccine contains less than 1 mmol (23 mg) of sodium per dose, which means it is essentially 'sodium-free'.
3. How to use Boostrix vaccine
- The Boostrix vaccine will be given as an intramuscular injection, never intravenously.
- You will receive a single dose of the vaccine.
- Your doctor will determine if you have been previously vaccinated against diphtheria, tetanus, and/or pertussis.
- Boostrix can be used if there is a suspicion of tetanus infection due to injury. Your doctor will also take additional measures, such as wound dressing and/or administration of tetanus antitoxin, to reduce the risk of disease
- Your doctor will inform you if a booster dose of the vaccine is necessary.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them. As with all injectable vaccines, there is a very small risk (up to 1 in 10,000 doses of the vaccine) of anaphylactic and anaphylactoid reactions. Such a condition can be recognized by the following symptoms:
- Rash that may be itchy or blistering,
- Swelling of the eyes and face,
- Difficulty breathing and swallowing,
- Sudden drop in blood pressure and loss of consciousness.Such reactions usually occur before leaving the doctor's office. However, in any such case, you should contact your doctor immediately.
Side effects that occurred during clinical trials in children aged 4 to 8 years:
Very common(may occur more frequently than 1 in 10 doses of the vaccine): pain, redness, and swelling at the injection site, irritability, drowsiness, fatigue.
Common(may occur up to 1 in 10 doses of the vaccine): loss of appetite, headache, fever equal to or above 37.5 ° C (including above 39 ° C), extensive swelling of the limb into which the vaccine was administered, vomiting, and diarrhea.
Uncommon(may occur up to 1 in 100 doses of the vaccine): upper respiratory tract infection, attention disorders, eye discharge and itching, conjunctivitis, rash, hard lump at the injection site, pain.
Side effects that occurred during clinical trials in adults, adolescents, and children aged 10 and over:
Very common(may occur more frequently than 1 in 10 doses of the vaccine): pain, redness, and swelling at the injection site, headache, fatigue, general malaise.
Common(may occur up to 1 in 10 doses of the vaccine): fever equal to or above 37.5 ° C, dizziness, nausea, hard lump or abscess at the injection site.
Uncommon(may occur up to 1 in 100 doses of the vaccine): fever above 39 ° C, pain, muscle and joint stiffness, vomiting, diarrhea, joint stiffness, muscle pain, itching, increased sweating, rash, swelling of the lymph nodes in the neck, armpits, or groin (lymphadenopathy), sore throat and difficulty swallowing (pharyngitis), upper respiratory tract infection, cough, fainting, flu-like symptoms such as fever, sore throat, runny nose, cough, and chills.
The following side effects have been reported during routine use of the Boostrix vaccine and are not specific to any age group: swelling of the face, lips, mouth, tongue, or throat, which may cause difficulty swallowing or breathing (angioedema), fainting or loss of consciousness, seizures (with or without fever), blisters (hives), unusual weakness (asthenia).
After administration of tetanus vaccines, transient neurological inflammation (causing pain, weakness, and paralysis of the limbs, often including the chest and face) has been very rarely reported (up to 1 in 10,000 doses of the vaccine) (Guillain-Barré syndrome).
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: 22 49 21 301
Fax: 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Boostrix vaccine
Keep out of the sight and reach of children. Do not use the vaccine after the expiry date stated on the carton and on the label of the pre-filled syringe after "EXP". The expiry date refers to the last day of the month stated. Store in a refrigerator (2 ° C – 8 ° C). Do not freeze. Freezing destroys the vaccine. Store in the original package to protect from light. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Boostrix vaccine contains
- The active substances of the vaccine are: Diphtheria toxoid not less than 2 international units (IU) (2.5 Lf) Tetanus toxoid not less than 20 international units (IU) (5 Lf) Bordetella pertussisantigens Pertussis toxoid 8 micrograms Filamentous hemagglutinin 8 micrograms Pertactin 2.5 micrograms
adsorbed on hydrated aluminum hydroxide (Al(OH)3)
0.3 milligrams of Al
and on aluminum phosphate (AlPO4)
0.2 milligrams of Al
Aluminum hydroxide and aluminum phosphate serve as adjuvants in this vaccine. Adjuvants are substances that are part of some vaccines and are intended to accelerate, enhance, and/or prolong the protective effect of the vaccine.
- Other ingredients are: sodium chloride and water for injections.
What Boostrix vaccine looks like and contents of the pack
Suspension for injection in a pre-filled syringe.
Boostrix is a white, slightly milky liquid in a pre-filled syringe (0.5 ml).
The Boostrix vaccine is available in a 1-dose pre-filled syringe, in packs of 1 or 10, with or without needles. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals S.A.
rue de l’Institut 89
1330 Rixensart, Belgium
Date of last revision of the leaflet:04/2023
Other sources of information
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
-------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Before administration, the vaccine should be at room temperature. The pre-filled syringe should be shaken vigorously to obtain a homogeneous, cloudy, white suspension.
Before administration, the vaccine should be inspected visually for the presence of any foreign particles and/or changes in the physical appearance of the vaccine. If any are found, the vaccine should not be administered.
Instructions for the pre-filled syringe
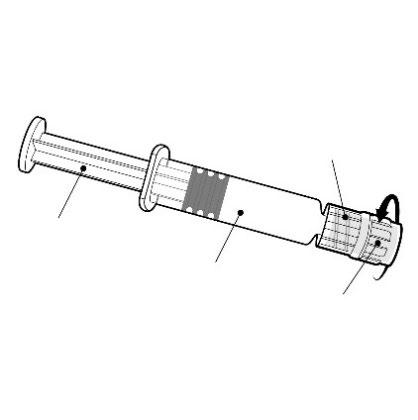
Hold the pre-filled syringe by the body, not by the plunger.
Unscrew the nozzle of the pre-filled syringe by turning it counterclockwise.
Luer Lock adapter
Plunger
Body
Nozzle
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
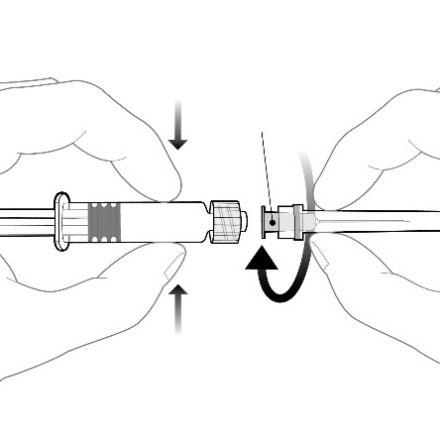
Attach the needle to the pre-filled syringe by screwing the needle nozzle onto the Luer Lock adapter (Luer Lock Adaptor, LLA) and turning it a quarter turn clockwise until the needle is locked.
Do not pull the plunger out of the body of the pre-filled syringe. If this happens, do not administer the vaccine.
Needle nozzle
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGlaxoSmithKline Biologicals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BoostrixDosage form: Suspension, 1 dose (0.5 ml)Active substance: pertussis, purified antigen, combinations with toxoidsPrescription requiredDosage form: Suspension, 1 dose (0.5 ml)Active substance: pertussis, purified antigen, combinations with toxoidsManufacturer: AJ Vaccines A/SPrescription requiredDosage form: Suspension, not less than 30 IU of diphtheria toxoid, not less than 40 IU of tetanus toxoid and not less than 4 IU of inactivated Bordetella pertussis strain suspension/0.5 ml; 1 day; 1 dose (0.5 ml)Active substance: pertussis, inactivated, whole cell, combinations with toxoidsPrescription required
Alternatives to Boostrix in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Boostrix in Ukraine
Alternative to Boostrix in Spain
Online doctors for Boostrix
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Boostrix – subject to medical assessment and local rules.














