
Binatta
Ask a doctor about a prescription for Binatta

How to use Binatta
LEAFLET INCLUDED IN THE PACKAGING
Leaflet included in the packaging: patient information
BINATTA, 25 mg, prolonged-release tablets
BINATTA, 50 mg, prolonged-release tablets
BINATTA, 100 mg, prolonged-release tablets
BINATTA, 150 mg, prolonged-release tablets
BINATTA, 200 mg, prolonged-release tablets
BINATTA, 250 mg, prolonged-release tablets
Tapentadol
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is BINATTA and what is it used for
- 2. Important information before taking BINATTA
- 3. How to take BINATTA
- 4. Possible side effects
- 5. How to store BINATTA
- 6. Contents of the pack and other information
1. What is BINATTA and what is it used for
Tapentadol – the active substance of BINATTA – is a strong pain-relieving opioid. BINATTA is indicated for the treatment of severe chronic pain in adults, where only opioid pain relief is appropriate.
2. Important information before taking BINATTA
When not to take BINATTA:
- if the patient is allergic to tapentadol or any of the other ingredients of this medicine (listed in section 6),
- in patients with asthma or dangerously slow and shallow breathing (respiratory depression, increased carbon dioxide levels in the blood),
- in patients with intestinal obstruction,
- in case of acute alcohol poisoning, sleeping pills, painkillers, or other psychotropic drugs (mood and emotion-affecting drugs) (see "BINATTA and other medicines").
Warnings and precautions
Before starting treatment with BINATTA, discuss with your doctor or pharmacist:
- in case of slow or shallow breathing,
- in case of increased intracranial pressure or impaired consciousness up to coma,
- in patients after head injury or with brain tumors,
- in patients with liver or kidney disease (see "How to take BINATTA"),
- in patients with pancreatic or biliary tract disease, including pancreatitis,
- in patients taking mixed agonist-antagonist opioid receptor drugs (e.g., pentazocine, nalbuphine) or partial opioid receptor agonists (e.g., buprenorphine),
- in patients with a history of seizure or taking other medications that increase the risk of seizures and may increase the risk of seizures,
- in case of a family history of substance abuse or dependence (addiction),
- in patients who smoke tobacco,
- in patients who have had mood problems (depression, anxiety disorders, or personality disorders) or have been treated by a psychiatrist for other mental illnesses.
This medicine contains tapentadol, which is an opioid. Repeated use of opioid painkillers can lead to decreased efficacy (tolerance) and may cause dependence and abuse, which can result in life-threatening overdose. If there is concern about dependence on BINATTA, consult a doctor. Taking the medicine (even at therapeutic doses) can lead to physical dependence, which may result in withdrawal symptoms and relapse of problems if treatment is suddenly stopped.
BINATTA may cause physical and psychological dependence. If there is a tendency to abuse drugs or addiction, treatment should be short-term and under close medical supervision.
Sleep apnea
BINATTA may cause sleep apnea, such as sleep apnea (pauses in breathing during sleep) and hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, waking up at night due to shortness of breath, difficulty maintaining sleep continuity, or excessive daytime sleepiness. If such symptoms are observed, consult a doctor. The doctor may consider reducing the dose of the medicine.
BINATTA and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
- The risk of side effects increases if you take medicines that can cause seizures (epileptic fits), such as antidepressants or antipsychotics. The risk of a seizure may increase if you take BINATTA at the same time. Your doctor will inform you if taking BINATTA is suitable for you.
- Concomitant use of BINATTA and sedatives, such as benzodiazepines or related drugs (some sleeping pills or sedatives, e.g., barbiturates) or painkillers, such as opioids, morphine, and codeine (also as a cough medicine), antipsychotics, antihistamines H1, alcohol, increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can be life-threatening. Therefore, concomitant use of such medicines should only be considered when other treatment options are not possible. However, if your doctor prescribes BINATTA in combination with sedative medicines, they should limit the dose and duration of concomitant treatment with such medicines. Concomitant use of opioids and medications used to treat epilepsy, neuropathic pain, or anxiety disorders (gabapentin and pregabalin) increases the risk of opioid overdose, respiratory depression, and can be life-threatening. Inform your doctor about taking gabapentin, pregabalin, or sedatives and strictly follow their instructions. It is worth informing friends or relatives about the possibility of the above-mentioned symptoms and consulting a doctor if they occur.
Opioids and gabapentin or pregabalin should be used with caution and the patient should be closely monitored for symptoms such as sedation, somnolence, and respiratory depression, as well as the potential for abuse and dependence. The patient should be advised to report any symptoms to their doctor and to seek medical attention immediately if they experience any of the above symptoms.
- If you are taking medicines that affect serotonin levels (e.g., some antidepressants), consult your doctor before taking BINATTA, due to the possibility of serotonin syndrome. Serotonin syndrome is rare but can be life-threatening. Its symptoms include: uncontrolled, rhythmic muscle contractions, including those that control eye movements, agitation, excessive sweating, tremors, excessive reflexes, including increased muscle tone and body temperature above 38°C. Your doctor can provide more information on this.
- Concomitant use of BINATTA with opioid receptor mixed agonist/antagonist drugs (e.g., pentazocine, nalbuphine) or partial agonists (e.g., buprenorphine) has not been studied. It is possible that BINATTA may not work properly if taken concomitantly with medicines from the above groups. Immediately inform your doctor about taking any of the above medicines.
- Concomitant use of BINATTA with strong inhibitors or inducers (e.g., rifampicin, phenobarbital, St. John's Wort) of certain enzymes necessary for the elimination of tapentadol from the body may affect the action of tapentadol or cause side effects, especially when starting or stopping their administration. Tell your doctor about all medicines you are currently taking.
- BINATTA should not be used concomitantly with MAO inhibitors (medicines used to treat depression). Tell your doctor if you have taken these medicines in the last 14 days.
BINATTA with food, drink, and alcohol
Do not drink alcohol while taking BINATTA, as some side effects, such as drowsiness, may worsen. The medicine can be taken with or without food.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Do not take BINATTA:
- during pregnancy, unless your doctor decides to prescribe it. Long-term use of tapentadol during pregnancy may cause withdrawal symptoms in newborns, which can be life-threatening if not recognized and treated by a doctor;
- while breastfeeding, as the medicine may be excreted in breast milk.
It is not recommended to take BINATTA:
- during childbirth, as it may cause dangerous slowing or shallowing of the newborn's breathing (respiratory depression).
Driving and using machines
BINATTA may cause drowsiness, dizziness, blurred vision, and affect reaction time. These symptoms may occur especially at the beginning of treatment with BINATTA, after the dose change prescribed by your doctor, or when taking alcohol or sedatives. Ask your doctor if you can drive or operate machinery after taking BINATTA. Your doctor will assess whether it is safe for you to drive or operate machinery.
3. How to take BINATTA
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Dosage should be adjusted according to the severity of the pain and the patient's individual sensitivity to pain.
As a rule, the smallest dose that effectively relieves the pain should be used.
Adults
Typically, the initial dose is 50 mg and is taken twice a day, approximately every 12 hours.
Total daily doses of BINATTA greater than 500 mg of tapentadol are not recommended.
Your doctor may prescribe a different, more suitable dose or interval between doses if necessary. If you feel that the effect of the medicine is too strong or too weak, consult your doctor or pharmacist.
Elderly patients
Dose adjustment is not usually necessary in elderly patients (over 65 years). Tapentadol elimination may be prolonged in this age group, and therefore, your doctor may prescribe a different dosing schedule.
Patients with impaired liver or kidney function
Patients with severe liver impairment should not take this medicine. In case of moderate liver impairment, your doctor will prescribe a different dosing schedule. Patients with mild liver impairment do not require dose adjustment.
Patients with severe kidney impairment should not take this medicine. In case of mild or moderate kidney impairment, dose adjustment is not necessary.
Use in children and adolescents
BINATTA is not recommended for use in children and adolescents below 18 years of age.
How to take BINATTA
BINATTA should be taken orally.
Swallow the tablet with a sufficient amount of liquid. The tablets must not be chewed or crushed
- this may lead to overdose due to the rapid release of the medicine in the body. The medicine can be taken with or without food.
The tablet can be divided into equal doses.
The empty tablet shell may not be completely digested and may be present in the stool. Do not worry, as the medicine (active substance) has already been absorbed by the body, and only the tablet shell is present in the stool.
Instructions for opening the blister pack
This medicine is packaged in child-resistant, perforated blister packs, divided into single doses. The tablets cannot be pushed through the blister pack. Open the blister packs as follows:
- 1. Tear off a single dose along the perforated line of the blister pack.
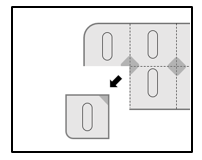
- 2. The intersection of the lines is an unprotected area.
- 3. Pull the unprotected part to tear off the top protective layer.
Duration of treatment with BINATTA
Do not take the tablets for longer than prescribed by your doctor.
Taking a higher dose of BINATTA than recommended
After taking very high doses, the following symptoms may occur:
- pupil constriction to the size of a pinhead,
- vomiting,
- blood pressure drop,
- rapid heartbeat,
- collapse, impaired consciousness, or coma (deep state of unconsciousness),
- seizures,
- dangerously slow or shallow breathing or respiratory arrest. In such cases, seek medical help immediately!
Missing a dose of BINATTA
If you miss a dose, your pain is likely to return. Do not take a double dose to make up for the missed dose. Continue with your original dosing schedule.
Stopping treatment with BINATTA
If you stop or discontinue treatment with BINATTA before the end of the treatment, your pain is likely to return. Consult your doctor before stopping the medicine.
As a rule, no side effects are observed after stopping the medicine; however, in rare cases, patients who have taken the medicine for some time and stop it abruptly may experience general malaise.
The following symptoms may occur:
- restlessness, tearfulness, runny nose, yawning, sweating, chills, muscle pain, and pupil dilation,
- irritability, anxiety, back pain, joint pain, weakness, abdominal cramps, difficulty sleeping, nausea, loss of appetite, vomiting, diarrhea, increased blood pressure, respiratory rate, and heart rate. If you experience any of these symptoms after stopping treatment, consult your doctor immediately. Do not stop taking BINATTA abruptly unless your doctor advises you to do so. Your doctor will tell you how to stop taking the medicine. Stopping the medicine may involve gradually reducing the dose.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, BINATTA can cause side effects, although not everybody gets them.
Important side effects or symptoms to watch out for and what to do if they occur:
- This medicine may cause allergic reactions. Symptoms may include wheezing, difficulty breathing, swelling of the eyelids, face, or lips, rash, or itching, especially affecting the whole body.
- Another serious side effect is excessive slowing and shallowing of breathing. This occurs most often in elderly and frail patients.
If any of these important side effects affect you, seek medical help immediately.
Other side effects that may occur:
Very common(may affect more than 1 in 10 people)
- nausea, constipation,
- dizziness, drowsiness, headache.
Common(may affect up to 1 in 10 people)
- loss of appetite, anxiety, depressive mood, sleep disorders, nervousness, restlessness,
- tremors, muscle spasms,
- flushing,
- shortness of breath,
- vomiting, diarrhea, indigestion,
- itching, excessive sweating, rash,
- feeling weak, tiredness, feeling of temperature change, dryness of mucous membranes, fluid retention (edema).
Uncommon(may affect up to 1 in 100 people)
- allergic reactions to the medicine (including skin swelling, hives, and in severe cases, difficulty breathing, low blood pressure, collapse, or shock),
- weight loss,
- disorientation, confusion, agitation (excitement), perception disorders, unusual dreams, euphoric mood, decreased level of consciousness, memory impairment, mental disorders,
- fainting, excessive sedation, balance disorders, speech disorders, tingling, abnormal skin sensations (e.g., burning, prickling),
- vision disorders,
- rapid heartbeat, slow heartbeat, palpitations, decreased blood pressure,
- abdominal discomfort,
- hives,
- urination disorders, frequent urination,
- sexual dysfunction,
- withdrawal syndrome (see "Stopping treatment with BINATTA"), feeling of abnormality, irritability.
Rare(may affect up to 1 in 1,000 people)
- dependence on the medicine, thinking disorders, seizures, feeling of impending fainting, coordination disorders,
- dangerously slow or shallow breathing (respiratory depression),
- delayed gastric emptying,
- feeling of intoxication, feeling of relaxation.
Frequency not known(cannot be estimated from the available data)
- delirium.
Generally, the risk of suicidal thoughts and behaviors is higher in patients with chronic pain. Additionally, medicines used to treat depression (which affect the neurotransmitter system in the brain) may increase this risk, especially at the beginning of treatment. Although tapentadol also affects neurotransmitters, data from human use have not provided evidence of an increased risk.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Ministry of Health: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store BINATTA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and blister after EXP.
The expiry date refers to the last day of the month stated.
There are no special precautions for storing the medicine.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What BINATTA contains
- The active substance is tapentadol.
BINATTA, 25 mg, prolonged-release tablets
Each prolonged-release tablet contains 25 mg of tapentadol (as phosphate).
BINATTA, 50 mg, prolonged-release tablets
Each prolonged-release tablet contains 50 mg of tapentadol (as phosphate).
BINATTA, 100 mg, prolonged-release tablets
Each prolonged-release tablet contains 100 mg of tapentadol (as phosphate).
BINATTA, 150 mg, prolonged-release tablets
Each prolonged-release tablet contains 150 mg of tapentadol (as phosphate).
BINATTA, 200 mg, prolonged-release tablets
Each prolonged-release tablet contains 200 mg of tapentadol (as phosphate).
BINATTA, 250 mg, prolonged-release tablets
Each prolonged-release tablet contains 250 mg of tapentadol (as phosphate).
- Other ingredients are: Tablet core:microcrystalline cellulose (E 460); hypromellose (E 464); colloidal anhydrous silica (E 551); magnesium stearate. Tablet coating:hypromellose (E 464); glycerol (E 422); talc (E 553b); microcrystalline cellulose (E 460); titanium dioxide (E 171); iron oxide red (E 172) (only for 25, 100, 150, 200, and 250 mg); iron oxide yellow (E 172) (only for 25, 100, and 200 mg); iron oxide black (E 172) (only for 25, 100, 150, 200, and 250 mg).
What BINATTA looks like and contents of the pack
BINATTA, 25 mg: brownish prolonged-release tablets with a longitudinal shape (6 mm x 12 mm) with a dividing line on both sides.
The tablet can be divided into equal doses.
BINATTA, 50 mg: white prolonged-release tablets with a longitudinal shape (6 mm x 13 mm) with a dividing line on both sides.
The tablet can be divided into equal doses.
BINATTA, 100 mg: yellowish prolonged-release tablets with a longitudinal shape (7 mm x 14 mm) with a dividing line on both sides.
The tablet can be divided into equal doses.
BINATTA, 150 mg: light red prolonged-release tablets with a longitudinal shape (7 mm x 15 mm) with a dividing line on both sides.
The tablet can be divided into equal doses.
BINATTA, 200 mg: yellow prolonged-release tablets with a longitudinal shape (8 mm x 16 mm) with a dividing line on both sides.
The tablet can be divided into equal doses.
BINATTA, 250 mg: reddish-brown prolonged-release tablets with a longitudinal shape (9 mm x 18 mm) with a dividing line on both sides.
The tablet can be divided into equal doses.
BINATTA is available in packs of:
BINATTA, 25 mg
20x1, 30x1, 40x1, 50x1, 54x1, 60x1, or 100x1 prolonged-release tablets in child-resistant, perforated blister packs..
BINATTA, 50–250 mg
20x1, 24x1, 30x1, 50x1, 54x1, 60x1, or 100x1 prolonged-release tablets in child-resistant, perforated blister packs
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer/importer
Marketing authorization holder:
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Germany
Manufacturer/importer:
Develco Pharma GmbH
Grienmatt 27
Farhnau
79650 Schopfheim
Germany
STADA Arzneimittel AG
Stadastrasse 2 – 18
61118 Bad Vilbel
Germany
Centrafarm Services B.V.
Van de Reijtstraat 31-E
4814 NE Breda
Netherlands
To obtain more detailed information on this medicine, contact the representative of the marketing authorization holder:
Stada Pharm sp. z o.o.
ul. Krakowiaków 44
02-255 Warsaw
Tel. +48 22 737 79 20
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany
Tapentadol AL 25 mg Retardtabletten
Tapentadol AL 50 mg Retardtabletten
Tapentadol AL 100 mg Retardtabletten
Tapentadol AL 150 mg Retardtabletten
Tapentadol AL 200 mg Retardtabletten
Tapentadol AL 250 mg Retardtabletten
Croatia
TAPISTA
Czech Republic
Taxemba
Denmark
Tapentadol STADA
Iceland
Tapentadol STADA 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg; forðahylki
Italy
Mudol
Netherlands
Tapentadol retard CF 25 mg, tabletten met verlengde afgifte
Tapentadol retard CF 50 mg, tabletten met verlengde afgifte
Tapentadol retard CF 100 mg, tabletten met verlengde afgifte
Tapentadol retard CF 150 mg, tabletten met verlengde afgifte
Tapentadol retard CF 200 mg, tabletten met verlengde afgifte
Tapentadol retard CF 250 mg, tabletten met verlengde afgifte
Norway
Tapentadol STADA
Poland
BINATTA
Slovakia
Tapestad retard 50 mg, 100 mg, 150 mg, 200 mg, 250 mg
Spain
Tapentadol retard STADA 25 mg comprimidos de liberación prolongada EFG
Tapentadol retard STADA 50 mg comprimidos de liberación prolongada EFG
Tapentadol retard STADA 100 mg comprimidos de liberación prolongada EFG
Tapentadol retard STADA 150 mg comprimidos de liberación prolongada EFG
Tapentadol retard STADA 200 mg comprimidos de liberación prolongada EFG
Tapentadol retard STADA 250 mg comprimidos de liberación prolongada EFG
Sweden
Tapentadol Depot STADA 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg; depottabletter
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterCentrafarm Services B.V. Develco Pharma GmbH STADA Arzneimittel AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BinattaDosage form: Tablets, 50 mgActive substance: tapentadolManufacturer: Krka, d.d., Novo mesto TAD Pharma GmbHPrescription requiredDosage form: Tablets, 100 mgActive substance: tapentadolManufacturer: Krka d.d. TAD Pharma GmbHPrescription requiredDosage form: Tablets, 150 mgActive substance: tapentadolManufacturer: Krka d.d. TAD Pharma GmbHPrescription required
Alternatives to Binatta in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Binatta in Spain
Alternative to Binatta in Ukraine
Online doctors for Binatta
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Binatta – subject to medical assessment and local rules.














