

Bibloc

Ask a doctor about a prescription for Bibloc

How to use Bibloc
Package Leaflet: Information for the Patient
Bibloc, 5 mg Coated Tablets
Bisoprolol Fumarate
Read the Package Leaflet Carefully Before Taking the Medication, as it Contains Important Information for the Patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medication has been prescribed for your specific condition. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet:
- 1. What is Bibloc and what is it used for
- 2. Important information before taking Bibloc
- 3. How to take Bibloc
- 4. Possible side effects
- 5. How to store Bibloc
- 6. Contents of the pack and other information
1. What is Bibloc and what is it used for
Bibloc belongs to a group of medicines called beta-blockers. They protect the heart from excessive activity.
Bibloc is used to treat:
high blood pressure
angina pectoris (chest pain)
heart failure, which causes shortness of breath during exertion or fluid retention in the body. In this case, Bibloc may be used as additional treatment for heart failure, treated with other medications.
2. Important information before taking Bibloc
When not to take Bibloc
If you are allergic to bisoprolol fumarate or any of the other ingredients of this medicine (listed in section 6).
If you have cardiogenic shock - a severe heart disorder with rapid, weak pulse, low blood pressure, cold, sweaty skin, weakness, and fainting.
If you have ever had wheezing or severe asthma, as breathing difficulties may occur.
If you have a slow heart rate (less than 60 beats per minute). If in doubt, consult your doctor.
If you have very low blood pressure.
If you have severe circulatory problems (which can cause tingling in the fingers and toes or their paleness or blueness).
If you have severe heart rhythm disorders.
If you have suddenly developed heart failure or existing heart failure is uncontrolled and requires hospital treatment.
If you have a buildup of acids in the body, diagnosed as metabolic acidosis. Your doctor will provide information on this.
If you have a tumor of the adrenal gland (so-called pheochromocytoma) that is not being treated.
In case of any doubts, consult your doctor.
Warnings and precautions
Before starting to take Bibloc, consult your doctor:
if you have wheezing or difficulty breathing (asthma), you should also use bronchodilators. A higher dose of beta-2-mimetic may be necessary.
if you have diabetes. Bibloc tablets may mask the symptoms of low blood sugar (such as rapid heart rate, palpitations, or excessive sweating).
if you do not eat foods with a constant consistency.
if you are being treated for allergies. Bibloc may increase sensitivity to allergens and worsen the severity of hypersensitivity reactions. Adrenaline treatment may then be ineffective and may need to be administered in a higher dose.
if you have first-degree atrioventricular block (conduction disorders in the heart).
if you have Prinzmetal's angina (chest pain caused by coronary artery spasm).
if you have circulatory problems in your hands and feet.
if you are going to have surgery under anesthesia, inform your doctor, hospital staff, or dentist about the medications you are taking.
if you are taking calcium channel blockers, such as verapamil and diltiazem. Concurrent use is not recommended (see also "Other medicines and Bibloc").
if you have had psoriasis (a recurring disease with skin peeling and dry skin rash) in the past.
if you have a pheochromocytoma (adrenal gland tumor). Before prescribing Bibloc, your doctor will need to provide appropriate treatment.
if you have thyroid function disorders. Bisoprolol tablets may mask the symptoms of hyperthyroidism.
There is currently no therapeutic experience with the use of Bibloc in heart failure in the following patients:
- with type 1 diabetes treated with insulin
- with severe kidney disease
- with severe liver disease
- with certain heart diseases
- who have had a heart attack in the last 3 months.
Treatment of heart failure with Bibloc requires systematic medical monitoring. This is absolutely necessary, especially at the beginning and end of treatment.
Do not stop taking Bibloc abruptly without a compelling reason.
In patients with high blood pressure and angina pectoris with concomitant heart failure, treatment should not be stopped abruptly. The dose of the medicine should be gradually reduced, every week by half.
Consult your doctor if any of the described warnings apply to you or have applied in the past.
Bibloc and other medicines
Tell your doctor or pharmacist about all the medicines you are currently taking or have recently taken, as well as any medicines you plan to take. This also applies to medicines that are available without a prescription. Certain medicines cannot be taken at the same time as Bibloc, and others may require changes, such as dose adjustments.
In any case, inform your doctor about taking or receiving the following medicines in addition to Bibloc:
medicines used to control blood pressure or heart function disorders (such as amiodarone, amlodipine, clonidine, digitalis glycosides, diltiazem, disopyramide, felodipine, flecainide, lidocaine, methyldopa, moxonidine, phenytoin, propafenone, quinidine, rilmendine, verapamil);
sedatives and medicines used to treat psychosis (mental illness), such as barbiturates (also used to treat epilepsy), phenothiazines (also used to treat vomiting and nausea);
medicines used to treat depression, such as tricyclic antidepressants, MAO-A inhibitors;
medicines used for anesthesia during surgery (see also "Warnings and precautions");
certain painkillers (such as acetylsalicylic acid, diclofenac, indomethacin, ibuprofen, naproxen);
medicines used to treat asthma, nasal congestion, or certain eye diseases, such as glaucoma (increased pressure in the eyeball) or pupil dilation;
certain medicines used to treat shock (such as adrenaline, dobutamine, noradrenaline);
mefloquine (a medicine used to treat malaria);
rifampicin (an antibiotic);
ergotamine derivatives (used to treat migraines).
All these medicines and Bibloc may affect blood pressure and/or heart function.
insulin or other anti-diabetic medicines. There is a possibility of enhanced glucose-lowering effect and masking of low blood sugar symptoms.
Bibloc with alcohol
Alcohol may enhance dizziness and drowsiness caused by Bibloc. In this case, you should avoid drinking alcohol.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before taking this medicine. Bibloc may have a harmful effect on pregnancy and/or the fetus. The risk of premature birth, miscarriage, low blood sugar in the child, and slowing of the child's heart rate increases. The medicine may also affect the development of the child. Therefore, Bibloc should not be used during pregnancy.
It is not known whether bisoprolol passes into breast milk, so it is not recommended to take Bibloc during breastfeeding.
Driving and using machines
The medicine may cause fatigue, drowsiness, or dizziness. If these symptoms occur, do not drive or operate machinery. Keep in mind that such symptoms may occur, especially at the beginning of treatment, when changing the medicine, or when consuming alcohol.
Bibloc contains lactose and sodium
If you have been diagnosed with intolerance to some sugars, consult your doctor before taking the medicine.
This medicine contains less than 1 mmol (23 mg) of sodium per coated tablet, which means it is considered "sodium-free".
3. How to take Bibloc
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, consult your doctor or pharmacist.
Your doctor will tell you how many tablets to take. Take the medicine in the morning, before, during, or after breakfast. Swallow the tablet(s) with a small amount of water. Do not chew or crush the tablets.
High blood pressure/angina pectoris
Adults
Your doctor will determine the dose of the medicine individually.
The recommended initial dose is 5 mg once daily.
The usual dose for adults is 10 mg once daily. Your doctor may decide to increase or decrease this dose.
The maximum dose is 20 mg once daily.
Severe kidney or liver impairment
In patients with severe kidney impairment: the maximum dose is 10 mg per day.
Elderly
Dose adjustment is not usually necessary. Your doctor will start treatment with the lowest possible dose.
Heart failure (reduced heart pumping power)
Before starting to take Bibloc, you are usually already taking an ACE inhibitor, diuretic, or digitalis glycoside (a heart medicine and antihypertensive).
The dose of the medicine will be gradually increased until the appropriate dose for you is reached:
1.25 mg once daily for 1 week. If this dose is well tolerated, it can be increased to:
2.5 mg once daily for the next week. If this dose is well tolerated, it can be increased to:
3.75 mg once daily for the next week. If this dose is well tolerated, it can be increased to:
5 mg once daily for 4 consecutive weeks. If this dose is well tolerated, it can be increased to:
7.5 mg once daily for 4 consecutive weeks. If this dose is well tolerated, it can be increased to:
10 mg once daily (maintenance dose).
The maximum dose is 10 mg once daily.
Your doctor will determine the optimal dose for you based on, among other things, side effects.
After the first dose of 1.25 mg, your doctor will check your blood pressure, heart rate, and heart function.
Liver or kidney impairment
Your doctor will carefully increase the dose of the medicine.
Elderly
Dose adjustment is not usually necessary.
If you feel that the effect of Bibloc is too strong or too weak, inform your doctor or pharmacist.
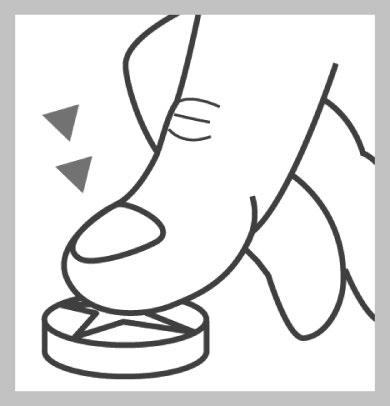
Place the tablet on a hard, flat surface, with the notch facing up.
Press your thumb on the center of the tablet. The tablet will break into two halves. After pressing on the center of each half, you will get 4 parts.
Duration of treatment
Bibloc is usually taken for a long time.
Use in children and adolescents
Due to the lack of studies on the effect of Bibloc in children and adolescents, its use is not recommended in this age group.
Taking a higher dose of Bibloc than recommended
In case of accidental ingestion of a higher dose than recommended, contact your doctor or pharmacist immediately.
Take the remaining tablets or this leaflet with youso that the medical staff knows what medicine has been taken. Symptoms of overdose may include dizziness, drowsiness, fatigue, shortness of breath and/or wheezing. Slow heart rate, low blood pressure, inadequate heart contractions, and low blood sugar (which can cause hunger, excessive sweating, and palpitations) may also occur.
Missing a dose of Bibloc
Do not take a double dose to make up for a missed dose. Take your usual dose as soon as you remember, and the next day return to your normal dosing schedule.
Stopping treatment with Bibloc
Do not stop taking Bibloc abruptly. If treatment is stopped abruptly, the disease may worsen. The dose of the medicine should be gradually reduced over several weeks, according to your doctor's instructions.
In case of any further doubts about the use of this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Bibloc can cause side effects, although not everybody gets them.
If a side effect is severe, occurs suddenly, or worsens rapidly, consult your doctor immediately to prevent serious reactions.
The most serious side effects are related to heart function:
slow heart rate (may occur more frequently than in 1 in 10 people)
worsening of existing heart failure (may occur less frequently than in 1 in 10 people)
slow or irregular heart rate (may occur less frequently than in 1 in 100 people)
If you experience dizziness, weakness, or difficulty breathing, consult your doctor as soon as possible.
If you experience severe hypersensitivity reactions, which may include swelling of the face, neck, tongue, lips, or throat, or difficulty breathing, consult your doctor immediately.
The following side effects are listed below by frequency of occurrence:
Common (may occur in less than 1 in 10 people):
fatigue. In the treatment of high blood pressure or angina pectoris, this side effect occurs infrequently.
dizziness, fatigue, and headache (especially at the beginning of treatment in patients with high blood pressure and angina pectoris; these symptoms are usually mild and often disappear within 1 to 2 weeks)
feeling of cold or numbness in the limbs (fingers or toes, ears, and nose); more frequent occurrence of cramping leg pain while walking
very low blood pressure (hypotension), especially in patients with heart failure
nausea, vomiting
diarrhea
constipation
Uncommon (may occur in less than 1 in 100 people):
fatigue. In the treatment of heart failure, this side effect occurs frequently.
decrease in blood pressure when standing up, which may cause dizziness, drowsiness, or fainting
sleep disorders
depression
irregular heart rate
breathing difficulties in patients with asthma or a history of breathing disorders
muscle weakness and cramps
Rare (may occur in less than 1 in 1000 people):
nightmares
hallucinations
fainting
hearing impairment
nasal congestion, causing a runny nose with irritation
skin allergic reactions (such as itching, sudden redness of the skin, rash)
dryness of the eyes due to reduced tear secretion (which can be very troublesome for contact lens wearers)
liver inflammation, causing abdominal pain, loss of appetite, and sometimes jaundice with yellowing of the eyes and skin, as well as dark urine
reduced sexual potency (impotence)
increased levels of lipids in the blood (triglycerides) and increased activity of liver enzymes
Very rare (may occur in less than 1 in 10,000 people):
worsening of psoriasis symptoms or occurrence of a similar, dry, flaky skin rash, as well as hair loss
itching or redness of the eyes (conjunctivitis)
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw
tel.: + 48 22 49 21 301/fax: + 48 22 49 21 309/website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Bibloc
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month stated.
Do not use the medicine in the bottle for more than 6 months after first opening.
Blister: No special precautions for storage.
Bottle: No special precautions for storage.
After first opening: Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Bibloc contains
The active substance is bisoprolol fumarate. Each coated tablet contains 5 mg of bisoprolol fumarate.
The other ingredients are: calcium hydrogen phosphate anhydrous, microcrystalline cellulose, maize starch, croscarmellose sodium, colloidal anhydrous silica, magnesium stearate, lactose monohydrate, hypromellose, titanium dioxide (E171), macrogol 4000, yellow iron oxide (E172).
What Bibloc looks like and contents of the pack
Yellow, round, coated tablets with a notch, marked "BIS 5" on one side.
The tablets can be divided into four equal doses.
The coated tablets are packaged in blisters of OPA/Aluminum/PVC/Aluminum foil or in HDPE bottles and placed in a cardboard box.
Blisters contain 30, 60, or 90 tablets.
Bottles contain 100 tablets.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
Manufacturer
Lek Pharmaceuticals d.d.
Verovškova 57
1526 Ljubljana, Slovenia
Lek S.A.
ul. Domaniewska 50 C
02-672 Warsaw
Rowa Pharmaceuticals Ltd.
Newtown, Bantry, Co. Cork, Ireland
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
D-39179 Barleben, Germany
Lek S.A.
ul. Podlipie 16
95-010 Stryków, Poland
To obtain more detailed information about the medicine and its names in other European Economic Area countries, please contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
Phone: 22 209 70 00
Date of last update of the leaflet:03/2022
Sandoz logo
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BiblocDosage form: Tablets, 1.25 mgActive substance: bisoprololManufacturer: Lek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbHPrescription requiredDosage form: Tablets, 2.5 mgActive substance: bisoprololManufacturer: Lek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbHPrescription requiredDosage form: Tablets, 3.75 mgActive substance: bisoprololManufacturer: Lek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbHPrescription required
Alternatives to Bibloc in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Bibloc in Spain
Alternative to Bibloc in Ukraine
Online doctors for Bibloc
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bibloc – subject to medical assessment and local rules.









