

Bibloc

Ask a doctor about a prescription for Bibloc

How to use Bibloc
Leaflet attached to the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Bibloc (Bisoprolol Fumarate 2.5 mg Film-coated Tablets), 2.5 mg, film-coated tablets
Bisoprolol fumarate
Bibloc and Bisoprolol Fumarate 2.5 mg Film-coated Tablets are different trade names for the same drug.
You should read the leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Bibloc and what is it used for
- 2. Important information before taking Bibloc
- 3. How to take Bibloc
- 4. Possible side effects
- 5. How to store Bibloc
- 6. Contents of the packaging and other information
1. What is Bibloc and what is it used for
Bibloc belongs to a group of medicines called beta-blockers. They protect the heart from excessive activity.
Bibloc is used to treat:
- heart failure, which causes shortness of breath during exercise or fluid retention in the body. In this case, Bibloc may be used as additional treatment for heart failure, which is treated with other medicines.
2. Important information before taking Bibloc
When not to take Bibloc
- If the patient is allergic to bisoprolol fumarate or any of the other ingredients of this medicine (listed in section 6).
- If the patient has cardiogenic shock - a severe heart disorder with rapid, weak pulse, low blood pressure, cold, sweaty skin, weakness, and fainting.
- If the patient has ever had wheezing or severe asthma, as breathing difficulties may occur.
- If the patient has a slow heart rate (less than 60 beats per minute). In case of doubt, consult a doctor.
- If the patient has very low blood pressure.
- If the patient has severe circulatory disorders (which can cause tingling, pallor, or cyanosis of the fingers and toes).
- If the patient has severe heart rhythm disorders.
- If the patient has suddenly developed heart failure or existing heart failure that is not controlled and requires hospital treatment.
- If the patient has a metabolic acidosis. The doctor will provide information on this.
- If the patient has a pheochromocytoma (a tumor of the adrenal gland) that is not being treated.
In case of any doubts, consult a doctor.
Warnings and precautions
Before starting to take Bibloc, you should consult a doctor:
- If the patient has wheezing or difficulty breathing (asthma), they should also use bronchodilators. A higher dose of beta-2-mimetic may be necessary.
- If the patient has diabetes. Bibloc tablets may mask the symptoms of low blood sugar (such as rapid heart rate, palpitations, or excessive sweating).
- If the patient does not eat foods with a fixed consistency.
- If the patient is being treated for allergies. Bibloc may increase sensitivity to allergens and worsen the severity of hypersensitivity reactions. Adrenaline treatment may not be effective and may need to be administered in a higher dose.
- If the patient has a first-degree atrioventricular block (conduction disorders in the heart).
- If the patient has Prinzmetal's angina (chest pain caused by coronary artery spasm).
- If the patient has circulatory disorders in the hands and feet.
- If the patient is to undergo surgery under anesthesia, they should inform their doctor, hospital staff, or dentist about the medications they are taking.
- If the patient has (or has had) psoriasis (a recurring disease characterized by skin peeling and dry rash).
- If the patient has a pheochromocytoma (a tumor of the adrenal gland). Before prescribing Bibloc, the doctor will need to provide appropriate treatment.
- If the patient has thyroid function disorders. Bisoprolol tablets may mask the symptoms of hyperthyroidism.
There is currently no therapeutic experience with the use of Bibloc in heart failure in the following patients:
- with type 1 diabetes, treated with insulin,
- with severe kidney disease,
- with severe liver disease,
- with certain heart diseases,
- who have had a heart attack in the last 3 months.
Treatment of heart failure with Bibloc requires systematic medical monitoring. This is absolutely necessary, especially at the beginning of treatment and after its completion.
Taking Bibloc should not be stopped suddenly without a compelling reason.
Consult a doctor if any of the described warnings apply to the patient or have occurred in the past.
Bibloc and other medicines
Tell your doctor or pharmacist about all the medicines you are currently taking or have recently taken, as well as any medicines you plan to take. This includes medicines that are available without a prescription. Certain medicines should not be taken at the same time as Bibloc, and others may require changes, such as dose adjustments.
In any case, inform your doctor about taking or receiving the following medicines in addition to Bibloc:
- medicines used to control blood pressure or heart function disorders (such as amiodarone, amlodipine, clonidine, digitalis glycosides, diltiazem, disopyramide, felodipine, flecainide, lidocaine, methyldopa, moxonidine, phenytoin, propafenone, quinidine, rilmenidine, verapamil);
- sedatives and medicines used to treat psychosis (a mental illness), such as barbiturates (also used to treat epilepsy), phenothiazines (also used to treat vomiting and nausea);
- medicines used to treat depression, such as tricyclic antidepressants, MAO-A inhibitors;
- medicines used for anesthesia during surgery (see also "Warnings and precautions");
- certain painkillers (such as acetylsalicylic acid, diclofenac, indomethacin, ibuprofen, naproxen);
- medicines used to treat asthma, nasal congestion, or certain eye diseases, such as glaucoma (increased pressure in the eyeball) or pupil dilation;
- certain medicines used to treat shock (such as adrenaline, dobutamine, noradrenaline);
- mefloquine (a medicine used to treat malaria);
- rifampicin (an antibiotic);
- ergotamine derivatives (used to treat migraines). All these medicines and Bibloc may affect blood pressure and/or heart function.
- insulin or other anti-diabetic medicines. There is a possibility of increased glucose-lowering effect and masking of low blood sugar symptoms.
Bibloc and alcohol
Alcohol may increase dizziness and drowsiness caused by Bibloc. In this case, you should avoid drinking alcohol.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before taking this medicine. Bibloc may harm the pregnancy and/or the fetus. The risk of premature birth, miscarriage, low blood sugar in the child, and slowing of the child's heart rate increases. The medicine may also affect the development of the child. Therefore, Bibloc should not be taken during pregnancy.
It is not known whether bisoprolol passes into breast milk, so it is not recommended to take Bibloc while breastfeeding.
Driving and operating machinery
The medicine may cause fatigue, drowsiness, or dizziness. If these symptoms occur, do not drive or operate machinery. Keep in mind that such symptoms may occur, especially at the beginning of treatment, when changing the medicine, or when consuming alcohol.
Bibloc contains lactose monohydrate and sodium
If you have been diagnosed with intolerance to some sugars, you should contact your doctor before taking the medicine.
This medicine contains less than 1 mmol (23 mg) of sodium per film-coated tablet, which means it is considered "sodium-free".
3. How to take Bibloc
This medicine should always be taken according to the doctor's or pharmacist's recommendations. In case of doubts, consult a doctor or pharmacist.
The doctor will inform you how many tablets to take. The medicine should be taken in the morning, before, during, or after breakfast. The tablet(s) should be swallowed with a small amount of water. Do not chew or crush the tablets.
The usual dose is:
Heart failure (reduced heart contraction force)
Before starting to take Bibloc, the patient usually already takes an ACE inhibitor, diuretic, or digitalis glycoside (a heart medicine and antihypertensive).
The dose of Bibloc will be gradually increased until the appropriate dose for the patient is reached:
1.25 mg once a day for 1 week. If this dose is well tolerated, it can be increased to:
2.5 mg once a day for the next week. If this dose is well tolerated, it can be increased to:
3.75 mg once a day for the next week. If this dose is well tolerated, it can be increased to:
5 mg once a day for 4 consecutive weeks. If this dose is well tolerated, it can be increased to:
7.5 mg once a day for 4 consecutive weeks. If this dose is well tolerated, it can be increased to:
10 mg once a day (maintenance dose).
The maximum dose is 10 mg once a day.
The doctor will determine the optimal dose for the patient based on, among other things, side effects.
After the first dose of 1.25 mg, the doctor will check the patient's blood pressure, heart rate, and heart function.
Liver or kidney function disorders
The doctor will increase the dose of Bibloc with caution.
Elderly
Dose adjustment is usually not necessary.
If you feel that the effect of Bibloc is too strong or too weak, tell your doctor or pharmacist.
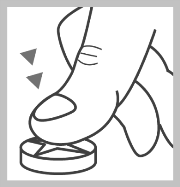
Place the tablet on a hard, flat surface, with the notch facing up.
Press your thumb on the center of the tablet. The tablet will break in half.
Duration of treatment
Bibloc is usually taken for a long time.
Use in children and adolescents
Due to the lack of studies on the effect of Bibloc in children and adolescents, its use is not recommended in this age group.
Taking a higher dose of Bibloc than recommended
In case of unintentional ingestion of a higher dose than recommended, contact a doctor or pharmacist immediately. Take the remaining tablets or this leaflet with you, so that medical personnel know what medicine has been taken. Symptoms of overdose may include dizziness, drowsiness, fatigue, shortness of breath and/or wheezing. There may also be slow heart rate, low blood pressure, inadequate heart contraction force, and low blood sugar (which can cause hunger, excessive sweating, and palpitations).
If the patient experiences dizziness, weakness, or difficulty breathing, they should contact a doctor as soon as possible.
If the patient experiences severe hypersensitivity reactions, which may include facial swelling, neck, tongue, lips, or throat, or difficulty breathing, they should contact a doctor immediately.
The following side effects are listed below by frequency of occurrence:
Common (may occur in less than 1 in 10 people):
- fatigue, exhaustion
- dizziness
- headache
- feeling of cold or numbness in the fingers of the hands or feet, ears, and nose; more frequent occurrence of cramping pain in the legs when walking
- very low blood pressure (hypotension), especially in patients with heart failure
- nausea, vomiting
- diarrhea
- constipation
Uncommon (may occur in less than 1 in 100 people):
- decrease in blood pressure when standing up, which may cause dizziness, drowsiness, or fainting
- sleep disorders
- depression
- irregular heart rate
- difficulty breathing in patients with asthma or a history of breathing disorders
- muscle weakness and cramps
Rare (may occur in less than 1 in 1000 people):
- nightmares
- hallucinations
- fainting
- hearing impairment
- rhinitis, causing a runny nose with irritation
- allergic skin reactions (such as itching, sudden redness of the skin, rash)
- dryness of the conjunctiva due to reduced tear secretion (which can be very troublesome in patients wearing contact lenses)
- hepatitis, causing abdominal pain, loss of appetite, and sometimes jaundice with yellowing of the eyes and skin, as well as dark urine
- decreased sexual potency (impotence)
- increased lipid levels in the blood (triglycerides) and increased liver enzyme activity
Very rare (may occur in less than 1 in 10,000 people):
- worsening of psoriasis symptoms or occurrence of a similar, dry, flaky rash, as well as hair loss
- itching or redness of the eyes (conjunctivitis)
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help gather more information on the safety of the medicine.
5. How to store Bibloc
- The medicine should be stored out of sight and reach of children.
- Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
- There are no special precautions for storage.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Bibloc contains
The active substance is bisoprolol fumarate. Each film-coated tablet contains 2.5 mg of bisoprolol fumarate.
The other ingredients are: calcium hydrogen phosphate, microcrystalline cellulose, maize starch, sodium croscarmellose, silicon dioxide, magnesium stearate.
Coating: lactose monohydrate, hypromellose, macrogol 4000, titanium dioxide (E 171).
What Bibloc looks like and what the packaging contains
White, round film-coated tablets with a notch, marked "BIS 2.5" on one side. The tablets can be divided into equal doses .
OPA/Al/PVC/Al blisters in a cardboard box containing 28 film-coated tablets.
For more detailed information, please contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in the UK, the country of export:
Sandoz Limited
Park View, Riverside Way
Watchmoor Park
Camberley, Surrey
GU15 3YL
United Kingdom
Manufacturer:
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
D-39179, Barleben, Germany
Rowa Pharmaceuticals Ltd.
Newtown, Bantry, Co. Cork, Ireland
Lek Pharmaceuticals d.d.
Verovškova 57, 1526 Ljubljana, Slovenia
Lek S.A.
ul. Domaniewska 50 C
02-672 Warsaw, Poland
Lek S.A.
ul. Podlipie 16 C
95-010 Stryków, Poland
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
UK license number: PL 04416/0924
Parallel import license number: 286/17 Date of leaflet approval: 05.09.2022
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Sandoz Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BiblocDosage form: Tablets, 1.25 mgActive substance: bisoprololManufacturer: Lek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbHPrescription requiredDosage form: Tablets, 2.5 mgActive substance: bisoprololManufacturer: Lek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbHPrescription requiredDosage form: Tablets, 3.75 mgActive substance: bisoprololManufacturer: Lek Pharmaceuticals d.d. LEK S.A. LEK S.A. Rowa Pharmaceuticals Ltd. Salutas Pharma GmbHPrescription required
Alternatives to Bibloc in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Bibloc in Spain
Alternative to Bibloc in Ukraine
Online doctors for Bibloc
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bibloc – subject to medical assessment and local rules.









