
Betoptic 0,5%

Ask a doctor about a prescription for Betoptic 0,5%

How to use Betoptic 0,5%
Leaflet attached to the packaging: information for the user
Warning!
Keep the leaflet. Information on the immediate packaging in a foreign language.
Betoptic 0.5%
5 mg/ml (0.5% w/v), eye drops, solution Betaxolol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Betoptic 0.5% and what is it used for
- 2. Important information before using Betoptic 0.5%
- 3. How to use Betoptic 0.5%
- 4. Possible side effects
- 5. How to store Betoptic 0.5%
- 6. Contents of the packaging and other information
1. What is Betoptic 0.5% and what is it used for
Betoptic 0.5% is used to treat high pressure in the eye in patients with chronic open-angle glaucoma or ocular hypertension.
High pressure in the eye
The eyes contain a clear fluid that nourishes the inside of the eye. This fluid drains out of the eye and is replaced by newly produced fluid. If the production of new fluid exceeds the drainage of fluid from the eye, the pressure in the eye increases. Too high pressure in the eye can damage vision.
Betoptic 0.5% belongs to a group of medicines used in glaucoma, called beta-adrenergic blockers.
This medicine is effective in reducing the pressure of the fluid in the eye. It can be used alone or in combination with other medicines that also reduce intraocular pressure.
2. Important information before using Betoptic 0.5%
When not to use Betoptic 0.5%:
- If the patient is allergicto betaxolol or any of the other ingredients of the medicine (listed in section 6).
- If the patient has heart disease,such as heart failure, bradycardia (very slow heart rate), sick sinus syndrome, atrioventricular block, or cardiogenic shock.
Page 1 of 7
- If the patient currently has or has had any respiratory problemssuch as severe asthma, severe chronic obstructive pulmonary disease (severe lung disease that can cause shortness of breath, difficulty breathing, and/or prolonged coughing).
Warnings and precautions
Before starting treatment with Betoptic 0.5%, the patient should discuss with their doctor or pharmacist if they currently have or have had:
- ischemic heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, or first-degree atrioventricular block, or low blood pressure. If the first symptoms of heart failure occur, the doctor will decide whether to discontinue Betoptic 0.5%,
- respiratory problems, asthma, or chronic obstructive pulmonary disease,
- peripheral vascular disease, such as Raynaud's disease or Raynaud's syndrome,
- diabetes, hypoglycemia, as betaxolol may mask the symptoms of low blood sugar,
- hyperthyroidism, as betaxolol may mask its symptoms,
- myasthenia (chronic muscle weakness),
- angle-closure glaucoma. Betoptic 0.5% should not be used as monotherapy in such cases,
- severe allergic reactions. The patient may be more sensitive to exposure to allergens. If a severe allergic reaction occurs during treatment with Betoptic 0.5% (skin rash, redness, and itching of the eye, fever, swelling of the throat, tongue, or face), treatment should be discontinued and medical attention should be sought immediately. Adrenaline treatment may not be as effective. If the patient is taking any other treatment, they should inform their doctor about the use of Betoptic 0.5%.
If the patient is scheduled for surgery, they should inform their doctor about the use of Betoptic 0.5%, as betaxolol may affect the effectiveness of certain medicines used during anesthesia.
If the patient has corneal disease, they should consult their doctor, as Betoptic 0.5% may cause dry eyes.
If the patient has had glaucoma surgery, they should consult their doctor before starting treatment with Betoptic 0.5%.
Betoptic 0.5% and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Betoptic 0.5% may affect the action of other medicines taken at the same time, and other medicines may affect the action of Betoptic 0.5%. These include:
- oral calcium channel blockers,
- beta-adrenergic blockers,
- antiarrhythmic medicines (including amiodarone),
- cardiac glycosides,
- parasympathomimetics,
- guanethidine,
- medicines that reduce the storage of catecholamines in nerve endings, such as reserpine derivatives,
- adrenaline,
- medicines used for emotional, behavioral, or mental disorders.
Page 2 of 7
If other eye drops or ointments are used, a minimum of 5 minutes should be allowed between administration of the different medicines. Eye ointments should be used last.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Betoptic 0.5% should not be used if the patient is pregnant, unless the doctor considers it necessary. Betoptic 0.5% should not be used if the patient is breastfeeding. Betaxolol passes into human milk.
Driving and using machines
Betoptic has no or negligible influence on the ability to drive and use machines.
However, like other eye products, Betoptic 0.5% may cause temporary blurred vision or other visual disturbances that may affect the ability to drive and use machines. If the patient experiences blurred vision after administering the eye drops, they should not drive or operate machinery until their vision is clear.
Betoptic 0.5% contains benzalkonium chloride
The medicine contains 0.1 mg of benzalkonium chloride per milliliter (0.1 mg/ml).
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Contact lenses should be removed before administering the eye drops and not put back for at least 15 minutes. Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or disorders affecting the cornea (the transparent layer on the front of the eye). If abnormal sensations in the eye, stinging, or pain in the eye occur after using the medicine, the patient should consult their doctor.
During treatment, it may be necessary to perform a control examination of the intraocular pressure. The patient should follow the doctor's instructions.
3. How to use Betoptic 0.5%
This medicine should always be used exactly as prescribed by the doctor.
In case of doubt, the patient should consult their doctor or pharmacist.
The doctor will determine how long the patient should use the medicine.
Betoptic 0.5% is for ophthalmic use only.
Dosage for adults, including elderly patients
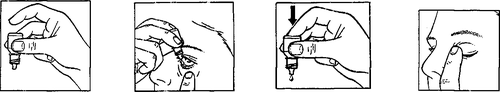
The recommended dose of Betoptic 0.5% is one drop into the conjunctival sac of the affected eye(s) twice daily, at regular intervals. However, the doctor may prescribe a different dosage, depending on the diagnosis. Below is a detailed instruction for use.
In some patients, stabilization of intraocular pressure may occur after several weeks of treatment with Betoptic 0.5%.
Page 4 of 7
The patient should notuse a double dose to make up for a forgotten dose.
If other eye drops or ointments are used, a minimum of 5 minutes should be allowed between administration of the different medicines. Eye ointments should be used last.
In case of accidental ingestion, the patient should consult their doctor.
In case of any further doubts about the use of this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Betoptic 0.5% can cause side effects, although not everybody gets them.
It is usually possible to continue using the eye drops, as long as the side effects are not severe. If the patient is concerned, they should consult their doctor or pharmacist. The patient should not stop using Betoptic 0.5% without consulting their doctor.
Topical beta-adrenergic blockers used in the eye may be absorbed into the systemic circulation. As a result, the same side effects may occur as with systemic beta-adrenergic blockers.
- Very common side effects (may affect more than 1 in 10 people):
- Eye disorders: eye discomfort.
- Common side effects (may affect up to 1 in 10 people):
- Eye disorders: blurred vision, increased tear production.
- General disorders: headache.
- Uncommon side effects (may affect up to 1 in 100 people):
- Eye disorders: inflammation of the surface of the eye with or without damage to the surface of the eye, conjunctivitis, blepharitis, visual disturbances, photophobia, burning and stinging of the eye, eye pain, dry eye, decreased visual acuity, eyelid disorders, itching of the eye, discharge from the eye, eyelid papillae, eye inflammation, eye irritation, conjunctival disorders, eye edema, eye redness.
- General disorders: slow heart rate, fast heart rate, asthma, shallow breathing, nausea, rhinitis.
- Rare side effects (may affect up to 1 in 1,000 people):
- Eye disorders: cataract.
- General disorders: fainting, unpleasant taste, cough, rhinitis, skin inflammation, rash, low blood pressure, anxiety, decreased libido.
The following side effects have been observed with other topical beta-adrenergic blockers used in the eye. They may also occur with Betoptic:
- Frequency not known (cannot be estimated from the available data):
- Eye disorders: eyelid redness, detachment of the retina, decreased corneal sensitivity, corneal erosion, eyelid ptosis, double vision.
- General disorders: hypersensitivity, allergic reactions that may include angioedema, urticaria, rash, itching, anaphylactic reactions, dizziness, stroke, cerebral ischemia, worsening of myasthenia symptoms (muscle weakness), paresthesia, arrhythmia, chest pain, palpitations, edema (fluid retention), congestive heart failure (heart disease characterized by shortness of breath and swelling of the feet and ankles), atrioventricular block, cardiac arrest, Raynaud's syndrome, feeling of cold hands and feet, bronchospasm, gastrointestinal disorders, diarrhea, dry mouth, abdominal pain, vomiting, hair loss, psoriasiform or exacerbation of psoriasis, muscle pain, sexual disorders, weakness, fatigue, insomnia, depression, nightmares, memory loss, hypoglycemia, heart failure.
Page 5 of 7
Like other topical ophthalmic medicines, betaxolol is absorbed into the systemic circulation. This may cause similar side effects as with oral and/or intravenous beta-adrenergic blockers. The frequency of systemic side effects after topical ophthalmic administration is lower than, for example, after oral or intravenous administration. The listed side effects also include side effects observed with other ophthalmic beta-adrenergic blockers.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Betoptic 0.5%
Betoptic 0.5% retains its potency for 4 weeks after first opening.
To prevent infections, the bottle should be discarded 4 weeks after first opening. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
The date of opening of the bottle should be written in the space provided below.
Opened:
The medicine should be kept out of the sight and reach of children.
Do not store above 25°C.
Store the bottle in the outer packaging.
Do not use Betoptic 0.5% after the expiry date stated on the packaging.
The expiry date refers to the last day of the month stated.
6. Contents of the packaging and other information
What Betoptic 0.5% contains
- The active substance is betaxolol 5 mg (as betaxolol hydrochloride).
- The other ingredients are benzalkonium chloride, disodium edetate, sodium chloride, sodium hydroxide, and/or hydrochloric acid (to adjust the pH), purified water.
What Betoptic 0.5% looks like and contents of the pack
Betoptic 0.5% is a clear, colorless solution supplied in a DROPTAINER bottle with a dropper.
A carton box contains 1 bottle of 5 ml.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Page 6 of 7
Marketing authorization holder in Greece, the country of export:
Immedica Pharma AB
Solnavägen 3H
113 63 Stockholm
Sweden
Manufacturer:
Siegfried El Masnou, S.A.
Camil Fabra 58, El Masnou
08320 Barcelona
Spain
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Marketing authorization number in Greece, the country of export: 34439/01-07-2003
8579/06-02-2007
87333/01-11-2022
Parallel import authorization number: 206/09
Date of approval of the leaflet: 14.10.2024
[information about the trademark]
Page 7 of 7
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Immedica Pharma AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Betoptic 0,5%Dosage form: Drops, 5 mg/mlActive substance: betaxololManufacturer: s.a. Alcon-Couvreur N.V. Siegfried El Masnou, S.A.Prescription requiredDosage form: Drops, 5 mg/mlActive substance: betaxololPrescription not requiredDosage form: Drops, 2.5 mg/mlActive substance: betaxololPrescription required
Alternatives to Betoptic 0,5% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Betoptic 0,5% in Ucrania
Alternative to Betoptic 0,5% in España
Online doctors for Betoptic 0,5%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Betoptic 0,5% – subject to medical assessment and local rules.














