
TIDIMAZ 20 mg/mL + 5 mg/mL Eye Drops Solution

How to use TIDIMAZ 20 mg/mL + 5 mg/mL Eye Drops Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Tidimaz 20 mg/ml + 5 mg/ml Eye Drops Solution
Dorzolamide/Timolol
Read the package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Tidimaz and what is it used for
- What you need to know before you use Tidimaz
- How to use Tidimaz
- Possible side effects
- Storing Tidimaz
- Contents of the pack and further information
1. What is Tidimaz and what is it used for
Tidimaz is a sterile solution without preservatives that contains two medicines: dorzolamide and timolol.
- Dorzolamide belongs to a group of medicines called "carbonic anhydrase inhibitors".
- Timolol belongs to a group of medicines called "beta-blockers".
These medicines reduce pressure in the eye in different ways.
Tidimaz is prescribed to reduce high pressure in the eye in the treatment of glaucoma when the use of a single beta-blocker eye drop is not appropriate.
2. What you need to know before you use Tidimaz
Do not useTidimaz
- if you are allergic to dorzolamide hydrochloride, timolol maleate, or any of the other ingredients of this medicine (listed in section 6).
- if you have now or have had in the past respiratory problems, such as asthma or severe chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, and/or persistent cough over a long time).
- if you have a slow heart rate, heart failure, or heart rhythm disturbances (irregular heartbeats).
- if you have severe kidney disease or a history of kidney stones.
- if you have excess acidity in the blood caused by an accumulation of chloride in the blood (hyperchloremic acidosis).
If you are not sure whether you should use Tidimaz, consult your doctor or pharmacist.
Warnings and Precautions
Consult your doctor before starting to use Tidimaz.
Tell your doctor about any medical or eye problems you have now or have had in the past, especially if you have:
- respiratory or lung problems, asthma, or chronic obstructive pulmonary disease.
- coronary heart disease (symptoms may include chest pain or tightness, difficulty breathing, or shortness of breath), heart failure, low blood pressure.
- heart rhythm disturbances such as a slow heart rate.
- circulatory problems (such as Raynaud's disease or Raynaud's syndrome).
- diabetes, as timolol may mask signs and symptoms of low blood sugar levels.
- thyroid problems, i.e., overactivity of the thyroid gland, as timolol may mask signs and symptoms.
- liver problems.
- any allergy or allergic reaction, including hives, swelling of the face, lips, tongue, and/or throat that can cause difficulty breathing or swallowing.
- muscle weakness or if you have been diagnosed with myasthenia gravis.
If you experience any of the following symptoms, stop usingthe eye drops and contact your doctor immediately:
- any eye irritation or any new eye problem, such as redness of the eyes or swelling of the eyelids, contact your doctor immediately.
- symptoms of an allergic reaction. See section 4 "Possible side effects" (e.g., skin rash or redness and itching of the eyes).
Tell your doctor that you are using Tidimaz if you have an eye injury or are going to have eye surgery, as timolol may affect the effects of some medicines used during anesthesia.
When Tidimaz is instilled in the eye, it can affect the whole body.
Tidimaz has not been studied in patients who use contact lenses. If you use soft contact lenses, you should consult your doctor before using Tidimaz. Before instilling this medicine, remove your contact lenses and reapply them after at least 15 minutes from instillation.
Children
There is limited experience with dorzolamide hydrochloride and timolol in infants and children.
Elderly Patients
In studies with dorzolamide hydrochloride and timolol with preservatives, the effects were similar in both elderly and younger patients.
Other Medicines and Tidimaz
Tidimaz may affect or be affected by other medicines you are using.
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including other eye drops or those bought without a prescription. This is especially important if you are taking:
- medicines to lower blood pressure or to treat heart diseases (such as calcium channel blockers, beta-blockers, or digoxin).
- medicines to treat an irregular heartbeat, such as calcium channel blockers, beta-blockers, or digoxin.
- monoamine oxidase inhibitors (MAOIs) used to treat depression.
- parasympathomimetic medicine that may have been prescribed to help you urinate. Parasympathomimetics are also a particular group of medicine that is sometimes used to help restore normal movement through the intestine.
- opioids such as morphine used to treat moderate to severe pain.
- medicines to treat diabetes.
- antidepressants known as fluoxetine and paroxetine.
- medicines with sulfonamide.
- quinidine (used to treat heart diseases and some types of malaria).
- other eye drops that also contain a beta-blocker or other carbonic anhydrase inhibitors like acetazolamide.
Athletes:
This medicine contains timolol, which may produce a positive result in doping tests.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
Do not use Tidimaz if you are pregnant. Tell your doctor if you are pregnant or planning to become pregnant.
Breastfeeding
Timolol may pass into breast milk. If treatment with Tidimaz is required, breastfeeding is not recommended. Tell your doctor if you are breastfeeding or planning to breastfeed.
Driving and Using Machines
No studies on the effects on the ability to drive and use machines have been performed. There are side effects associated with Tidimaz, such as blurred vision, that may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
3. How to Use Tidimaz
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, consult your doctor or pharmacist again.
The appropriate dosage and duration of treatment will be determined by your doctor.
The recommended dose is one drop in the affected eye(s) in the morning and at night.
If you are using Tidimaz at the same time as another eye drop, the drops should be instilled at least 10 minutes apart. Ophthalmic ointments should be administered last.
Do not change the dose of the medicine without consulting your doctor.
Tidimaz is a sterile solution that does not contain preservatives. See also Section 6 - Appearance of the product and contents of the pack.
Before instilling the eye drops:
- When using for the first time, before administering a drop in the eye, first practice using the dropper bottle by squeezing it slowly to administer a drop in the air, away from the eye.
- When you are confident that you can administer a drop, choose the position that you find most comfortable for instilling the drops (you can sit, lie on your back, or stand in front of a mirror).
Instructions for use:
- Wash your hands carefully before using this medicine.
- If the carton or bottle is damaged, you should not use the medicine.
- When using the medicine for the first time, you must unscrew the cap after making sure that the tamper-evident ring on the cap has not been broken. You should feel a slight resistance until the tamper-evident ring breaks (see Image 1).
- If the tamper-evident ring is loose, it should be discarded because it may fall into the eye and cause injury.
- Tilt your head back and gently pull down the lower eyelid to form a pocket between the eye and the eyelid (see Image 2). You should avoid contact between the tip of the bottle and the eyes, eyelids, or fingers to avoid contaminating the solution.
- Apply one drop into the pocket by gently squeezing the bottle. Squeeze the bottle gently in the middle and let one drop fall into the eye. There may be a delay of a few seconds between compression and the drop coming out (see Image 3). Do not squeeze too hard. If you are not sure how to administer this medicine, you should ask your doctor, pharmacist, or nurse.
- Close your eye and press the inner corner of the eye with your finger for about two minutes. This helps prevent the drop from reaching the rest of the body.
- Repeat steps 5, 6, and 7 in the other eye if your doctor has told you to do so.
- After use and before closing, shake the bottle once downwards without touching the dropper tip to remove any residual liquid from the tip. This is necessary to ensure the administration of subsequent drops. Screw the cap back on the bottle after application (see Image 4).
If a drop misses the eye, try again.
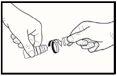

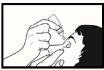
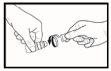
Image 1Image 2Image 3Image 4
If you use more Tidimaz than you should
If you apply too many drops in the eye or swallow some of the contents of the bottle, among other effects, you may feel dizziness, have difficulty breathing, or notice that your heart beats more slowly. Contact your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicological Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken. It is recommended to bring the packaging and the package leaflet of the medicine to the healthcare professional.
If you forget to use Tidimaz
It is important to use Tidimaz as your doctor has prescribed.
If you forget to apply a dose, you should apply it as soon as possible. However, if it is almost time for the next dose, do not apply the missed dose and continue with the scheduled dosing program as usual.
Do not use a double dose to make up for missed doses.
If you stop using Tidimaz
If you want to stop using this medicine, consult your doctor first.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you develop allergic reactions that include hives or rash with itching, localized or generalized rash, itching, swelling of the face, lips, tongue, and/or throat that can cause difficulty breathing or swallowing or chest pain, shortness of breath, sweating, feeling of anxiety, nausea (symptoms of a heart attack), you must stop using Tidimaz and talk to your doctor immediately.
The following side effects have been reported with dorzolamide hydrochloride and timolol maleate without preservatives or one of its components during clinical trials or during post-marketing experience:
Very common (may affect more than 1 in 10 people)
- Burning and stinging in the eyes, alteration of taste.
Common (may affect up to 1 in 10 people)
- redness in and around the eyes, tearing or itching of the eyes, corneal erosion (damage to the front layer of the eyeball), swelling and/or irritation in and around the eyes, feeling of having something in the eye, decreased sensitivity of the cornea (not noticing something entering the eye and not feeling pain), eye pain, dry eyes, blurred vision, headache, sinusitis (feeling of tension or congestion in the nose), feeling sick, also known as nausea, weakness/tiredness, fatigue.
Uncommon (may affect up to 1 in 100 people)
- dizziness, depression, inflammation of the iris, visual disturbances including refractive changes (due to the suspension of miotic therapy in some cases), difficulty breathing (dyspnea), slow heart rate, fainting, indigestion, kidney stones (often marked by a sudden onset of severe pain and cramps in the lower back and/or side, groin, or abdomen).
Rare (may affect up to 1 in 1,000 people)
- systemic lupus erythematosus (an immune system disease that can cause inflammation of internal organs), tingling or numbness of hands or feet, difficulty sleeping (insomnia), nightmares, memory loss, weakening of the muscles (increase in signs and symptoms of myasthenia gravis - muscle disorder), decreased sexual desire, stroke, temporary myopia that may resolve when treatment is discontinued, detachment of the layer under the retina that contains blood vessels after filtration surgery that can cause visual disturbances, drooping eyelids (making the eye stay half-closed), double vision, formation of crusts on the eyelids, swelling of the cornea (with symptoms of visual disturbances), low eye pressure, ringing in the ears, low blood pressure, irregular heartbeats (changes in the rhythm or speed of heartbeats), congestive heart failure (heart disease with difficulty breathing and swelling of feet and legs due to fluid accumulation), edema (fluid accumulation), palpitations (faster and/or irregular heartbeats), cerebral ischemia (reduced blood supply to the brain), chest pain, heart attack, Raynaud's phenomenon, swelling or coldness of hands and feet and reduced circulation in arms and legs, leg cramps and/or pain in the legs when walking (claudication), difficulty breathing, feeling of shortness of breath, nasal secretion or congestion, nasal bleeding, difficulty breathing (respiratory failure), constriction of airways in the lungs, cough, throat irritation, dry mouth, diarrhea, contact dermatitis, hair loss, psoriasis or worsening of psoriasis (skin rash with a silvery white appearance), Peyronie's disease (which can cause curvature of the penis), allergic reactions such as skin rash, hives, itching, in rare cases possible swelling of the lips, eyes, and mouth, wheezing or severe skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis).
Frequency not known (frequency cannot be estimated from the available data)
Difficulty breathing, feeling of a foreign body in the eye (feeling that something is in the eye), hallucinations, strong and/or irregular heartbeats (palpitations), increased heart rate, increased blood pressure.
As with other eye medicines, timolol is absorbed into the bloodstream. This may cause side effects similar to those seen with oral beta-blockers. The incidence of side effects after topical ophthalmic administration is lower than when medicines, for example, are taken orally or injected. Additional side effects listed include reactions observed within the class of beta-blockers when used to treat eye conditions:
Frequency not known (frequency cannot be estimated from the available data)
Low blood sugar levels, heart failure, a type of heart rhythm disorder, abdominal pain, vomiting, muscle pain not caused by exercise, sexual dysfunction.
Reporting of Side Effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tidimaz
This medication does not require special storage conditions.
Once opened, do not store at a temperature above 25 °C.
5 ml bottle:
Discard 60 days after the first opening of the bottle.
10 ml bottle:
Discard 90 days after the first opening of the bottle.
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the box and on the label of the bottle after CAD. The expiration date is the last day of the indicated month.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition ofTidimaz
- The active ingredients are dorzolamide and timolol. Each ml contains 20 mg of dorzolamide (as 22.26 mg of dorzolamide hydrochloride) and 5 mg of timolol (as 6.83 mg of timolol maleate).
Each drop (approximately 35 microliters (µl)) contains 0.70 mg of dorzolamide and 0.18 mg of timolol.
- The other components are hydroxyethylcellulose, mannitol, sodium citrate, sodium hydroxide, and water for injectable preparations.
Appearance of the product and package contents
Tidimaz is a sterile, colorless, transparent, and viscous liquid.
It is presented in white LDPE bottles (5 ml, 11 ml) with an HDPE multidose dropper applicator that prevents contamination of the contents thanks to a silicone valve system and filtered air return to the bottle, and an HDPE tamper-evident cap, and a cardboard box.
Package sizes: 1 x 5 ml, 3 x 5 ml, 1 x 10 ml, 2 x 10 ml.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
BRILL PHARMA, S.L.
C/ Munner, 8
08022 Barcelona
Spain
Manufacturer
Rafarm S.A.
Thesi Pousi Xatzi Agiou Louka
Paiania, 190 02
Greece
FRINO Pharm e.K.
Keplerweg 3
82538 Geretsried
Germany
This medication is authorized in the member states of the European Economic Area under the following names:
Czech Republic – Dorzolamide/Timolol Farmaprojects
Germany – Tidimaz 20 mg/ml Augentropfen, Lösung
Spain – Tidimaz 20 mg/ml eye drops, solution
Date of the last revision of this leaflet:June 2024
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.es/)
- Country of registration
- Average pharmacy price24.23 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TIDIMAZ 20 mg/mL + 5 mg/mL Eye Drops SolutionDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for TIDIMAZ 20 mg/mL + 5 mg/mL Eye Drops Solution
Discuss questions about TIDIMAZ 20 mg/mL + 5 mg/mL Eye Drops Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















