
Auxilen

Ask a doctor about a prescription for Auxilen

How to use Auxilen
Leaflet attached to the packaging: information for the user
Auxilen, 50 mg/2 mL, solution for injection/infusion
Dexketoprofen
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Auxilen and what is it used for
- 2. Important information before using Auxilen
- 3. How to use Auxilen
- 4. Possible side effects
- 5. How to store Auxilen
- 6. Contents of the packaging and other information
1. What is Auxilen and what is it used for
Auxilen is a pain-relieving medicine belonging to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs).
Auxilen is used for the symptomatic treatment of moderate to severe acute pain when oral administration is not appropriate, e.g. post-operative pain, pain in renal colic, and pain in the lumbosacral area.
2. Important information before using Auxilen
When not to use Auxilen:
- If the patient is allergic to dexketoprofen or any of the other ingredients of this medicine (listed in section 6);
- If the patient has hypersensitivity to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (NSAIDs);
- If the patient has asthma or has had asthma attacks in the past, acute allergic rhinitis (short-term inflammation of the nasal lining), nasal polyps (growths in the nasal passages caused by allergies), urticaria (hives), angioedema (swelling of the face, eyes, lips, tongue, or respiratory disorders) or wheezing after taking acetylsalicylic acid or other NSAIDs;
- If the patient has had hypersensitivity reactions to sunlight: photoallergic or phototoxic reactions (in particular, redness and/or blisters on the skin exposed to the sun) while taking ketoprofen (NSAID) or fibrates (medicines used to lower blood lipid levels);
- If the patient has stomach or duodenal ulcer disease or gastrointestinal bleeding or if they have had gastrointestinal bleeding, ulceration, or perforation in the past;
- If the patient has had gastrointestinal bleeding or perforation due to the use of NSAIDs;
- If the patient has certain chronic gastrointestinal diseases (e.g. indigestion, heartburn);
- If the patient has intestinal diseases with chronic inflammation (Crohn's disease or ulcerative colitis);
- If the patient has severe heart failure, moderate or severe renal impairment, or severe liver dysfunction;
- If the patient has a tendency to bleed or coagulation disorders;
- If the patient is severely dehydrated (excessive fluid loss from the body) due to vomiting, diarrhea, or inadequate fluid intake;
- If the patient is in the third trimester of pregnancy or breastfeeding.
Warnings and precautions
Before starting treatment with Auxilen, the patient should discuss the following with their doctor:
- If the patient has had chronic inflammatory bowel diseases (ulcerative colitis, Crohn's disease) in the past;
- If the patient has or has had other stomach or intestinal diseases;
- If the patient is taking other medicines that increase the risk of stomach ulcers (e.g. oral steroids, certain antidepressants (SSRI, e.g. serotonin reuptake inhibitors), anticoagulant medicines such as warfarin);
- If the patient has heart disease, has had a stroke, or is at risk of these conditions (e.g. high blood pressure, diabetes, high cholesterol, or smoking). In these cases, the patient should consult their doctor before taking this medicine. Taking such medicines as Auxilen may be associated with a small increased risk of heart attack (myocardial infarction) or stroke. This risk increases with long-term use of high doses of the medicine. The patient should not exceed the recommended dose and duration of treatment;
- If the patient is elderly: there is an increased risk of side effects (see section 4). In this case, the patient should consult their doctor immediately;
- If the patient has allergies or has had allergy problems in the past;
- If the patient has kidney, liver, or heart disorders (hypertension and/or heart failure) as well as fluid retention or if any of these problems have occurred in the past;
- In patients taking diuretics or in patients with decreased hydration and reduced blood volume due to excessive fluid loss (e.g. frequent urination, diarrhea, or vomiting);
- In women during the first and second trimester of pregnancy;
- If the patient has blood disorders or blood cell disorders;
- If the patient has systemic lupus erythematosus or mixed connective tissue disease (immune system disorders affecting connective tissue);
- If the patient has chickenpox, as non-steroidal anti-inflammatory drugs may rarely worsen the disease;
- If the patient has asthma and chronic rhinitis, chronic sinusitis, and/or nasal polyps, the risk of allergy to acetylsalicylic acid and/or NSAIDs is higher than in the rest of the population. Administration of this medicine may cause asthma attacks or bronchospasm, especially in patients allergic to acetylsalicylic acid and/or NSAIDs.
Children and adolescents
Auxilen has not been studied in children and adolescents. The safety and efficacy of this medicine have not been established, and therefore, it should not be used in children and adolescents.
Auxilen and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. Some medicines should not be taken at the same time, and in the case of other medicines, the dose may need to be changed due to their concurrent use.
The patient should always inform their doctor, dentist, or pharmacist if they are taking any of the following medicines with Auxilen:
- Acetylsalicylic acid (aspirin), corticosteroids, or other anti-inflammatory medicines;
- Warfarin, heparin, or other anticoagulant medicines;
- Lithium used to treat certain mood disorders;
- Methotrexate used to treat rheumatoid arthritis and cancer;
- Hydantoin derivatives and phenytoin used to treat epilepsy;
- Sulfamethoxazole used to treat bacterial infections.
Concomitant use that requires caution:
- ACE inhibitors, diuretics, beta-blockers, and angiotensin II antagonists used to treat high blood pressure and heart disease;
- Pentoxifylline and oxypentifylline used to treat ulcers in chronic venous insufficiency;
- Zidovudine used to treat viral infections;
- Aminoglycoside antibiotics used to treat bacterial infections;
- Chlorpropamide and glibenclamide used to treat diabetes.
Concomitant use that requires special consideration:
- Quinolone antibiotics (e.g. ciprofloxacin, levofloxacin) used to treat bacterial infections;
- Cyclosporine and tacrolimus used to treat immune system disorders and in transplants;
- Streptokinase and other thrombolytic or fibrinolytic medicines, i.e. medicines used to dissolve blood clots;
- Probenecid used to treat gout;
- Digoxin used to treat chronic heart failure;
- Mifepristone used as an abortifacient (to induce abortion);
- Antidepressant medicines from the group of selective serotonin reuptake inhibitors (SSRIs);
- Antiplatelet medicines used to reduce platelet aggregation and blood clot formation.
In case of any doubts about taking Auxilen with other medicines, the patient should consult their doctor or pharmacist.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor before using Auxilen.
Auxilen should not be used during the last three months of pregnancy or during breastfeeding.
Auxilen should not be given to pregnant women in the last 3 months of pregnancy, as it may harm the unborn child or cause problems during delivery. It may cause kidney and heart problems in the unborn child. It may increase the risk of bleeding in the mother and child and cause prolonged or delayed delivery. During the first 6 months of pregnancy, Auxilen should not be used unless the doctor considers it absolutely necessary. If treatment is necessary during this period or when trying to conceive, the lowest possible dose should be used for the shortest possible time. From
- the 20th week of pregnancy, Auxilen may cause kidney problems in the unborn child if taken for more than a few days. This may lead to a decrease in the amount of amniotic fluid surrounding the baby (oligohydramnios). If treatment is necessary for a longer period, the doctor may recommend additional monitoring.
Dexketoprofen may make it more difficult to get pregnant. The patient should inform their doctor if they plan to become pregnant or are having trouble getting pregnant.
Driving and using machines
Auxilen may cause dizziness and fatigue, and therefore, it may have a minor effect on the ability to drive vehicles and operate machinery. If the patient experiences such symptoms, they should not drive vehicles or operate machinery until the symptoms have resolved.
In case of any doubts, the patient should consult their doctor.
Auxilen contains ethanol and sodium
Each ampoule of Auxilen contains 200 mg of ethanol, which is equivalent to 5 mL of beer or 2.08 mL of wine per dose.
This may be harmful to individuals with alcohol dependence.
This should be taken into account in pregnant women, breastfeeding women, children, and high-risk groups such as patients with liver disease or epilepsy.
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means the medicine is essentially "sodium-free".
3. How to use Auxilen
This medicine should always be used exactly as prescribed by the doctor. In case of doubts, the patient should consult their doctor.
The doctor will inform the patient about the dose of Auxilen, which will depend on the type, severity, and duration of the symptoms. The recommended dose is 50 mg of dexketoprofen (1 ampoule of Auxilen) every 8 to 12 hours. If necessary, the dose can be repeated after 6 hours. The patient should not exceed the maximum daily dose, which is 150 mg of dexketoprofen (3 ampoules of Auxilen).
Auxilen is intended for short-term use and should only be used during the acute phase of pain (no longer than 2 days). The doctor will switch to oral pain-relieving medicines as soon as possible.
In elderly patients with renal impairment and in patients with kidney or liver disorders, the dose should not exceed 50 mg of Auxilen per day (equivalent to 1 ampoule).
Method of administration
Auxilen can be administered intramuscularly or intravenously (technical details for intravenous administration are provided in the section for healthcare professionals).
When administering Auxilen intramuscularly, the solution should be injected immediately after opening the ampoule, by slow injection into the muscle.
Only clear and colorless solutions should be used.
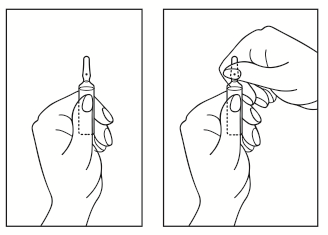
Instructions for opening the ampoule:
- 1) Turn the ampoule with the colored dot upwards. If there is a part of the solution in the upper part of the ampoule, gently tap the ampoule with your finger to bring the solution down to the lower part.
- 2) To open the ampoule, use both hands; holding the ampoule in one hand, use the other hand to remove the top part of the ampoule in the direction opposite to the colored dot (see the diagram below).
Use in children and adolescents
This medicine should not be used in children and adolescents (under 18 years of age).
Overdose of Auxilen
In case of suspected overdose, the patient should inform their doctor or pharmacist or go to the emergency department of the nearest hospital. The patient should take the packaging of this medicine or this patient information leaflet with them.
The patient should not take a double dose to make up for a missed dose. The next dose should be taken according to the dosing schedule (see section 3 "How to use Auxilen").
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Auxilen can cause side effects, although not everybody gets them.
Possible side effects are listed below and are grouped according to their frequency of occurrence.
Common side effects (may affect up to 1 in 10 people):
Nausea and/or vomiting, pain at the injection site, reactions at the injection site, e.g. inflammation, bruising, or bleeding.
Uncommon side effects (may affect up to 1 in 100 people):
Vomiting blood, low blood pressure, fever, blurred vision, dizziness, drowsiness, sleep disorders, headache, anemia, abdominal pain, constipation, indigestion, diarrhea, dry mouth, facial flushing, rash, skin inflammation, itching, increased sweating, fatigue, pain, feeling cold.
Rare side effects (may affect up to 1 in 1,000 people):
Stomach ulcers, gastrointestinal bleeding, or perforation, high blood pressure, fainting, slow breathing rate, superficial thrombophlebitis (vein inflammation due to blood clot), irregular heartbeat (extrasystoles), rapid heartbeat, swelling of limbs, throat swelling, abnormal sensations, feeling of increased body temperature, and tremors, ringing in the ears (tinnitus), itchy rash, jaundice, acne, back pain, kidney pain, increased urine output, menstrual disorders, benign prostatic hyperplasia, muscle stiffness, joint stiffness, muscle cramps, abnormal liver function tests (blood tests), high blood sugar levels (hyperglycemia), low blood sugar levels (hypoglycemia), high levels of certain fats in the blood (hypertriglyceridemia), sensation of pins and needles or tingling (paresthesia), increased excretion of ketone bodies or protein in the urine, liver cell damage (hepatitis), acute kidney failure.
Very rare side effects (may affect up to 1 in 10,000 people):
Anaphylactic reactions (acute allergic reactions that can lead to anaphylactic shock), skin ulcers, lips, eyes, and genital area (Stevens-Johnson syndrome and Lyell's syndrome), facial swelling or lip and throat swelling (angioedema), shortness of breath due to bronchospasm, pancreatitis, skin hypersensitivity reactions, and hypersensitivity to sunlight, kidney damage, decreased white blood cell count (neutropenia), decreased platelet count (thrombocytopenia).
The patient should inform their doctor if they notice any side effects, especially those related to the stomach or intestines (e.g. stomach pain, heartburn, or bleeding), if they have had similar side effects in the past due to long-term use of anti-inflammatory medicines, especially in elderly patients.
The patient should stop using Auxilen immediately if they experience a skin rash or any changes in the mucous membranes (e.g. inside the mouth) or any signs of an allergic reaction.
When using non-steroidal anti-inflammatory drugs (NSAIDs), fluid retention, and swelling (especially of the ankles and feet), increased blood pressure, and heart failure may occur.
Taking such medicines as Auxilen may be associated with a small increased risk of heart attack (myocardial infarction) or stroke.
In patients with systemic lupus erythematosus or mixed connective tissue disease (immune system disorders affecting connective tissue), NSAID administration may rarely cause fever, headache, and neck stiffness.
The patient should inform their doctor immediately if they experience signs of infection or worsening of their condition while taking Auxilen.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Auxilen
There are no special precautions for storage. Store in the original packaging to protect from light. Do not freeze.
The medicine should be stored out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton, after "Expiry date" (EXP). The expiry date refers to the last day of the month.
Auxilen is intended for single use, and any unused solution should be discarded.
Do not use this medicine if the patient notices that the solution is not clear and colorless and there are signs of contamination (e.g. particles).
It has been shown that the solution in 0.9% sodium chloride, 5% glucose, and Ringer's lactate solution, stored at 25°C and 2-8°C, maintains chemical stability for 18 hours, provided it is protected from daylight.
From a microbiological point of view, unless the dilution method excludes the risk of microbial contamination, the product should be used immediately. If not used immediately, the storage time and conditions are the responsibility of the user.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Auxilen contains
- -The active substance of the medicine is dexketoprofen. 1 mL of solution contains trometamol dexketoprofen equivalent to 25 mg of dexketoprofen. One ampoule (2 mL) contains trometamol dexketoprofen equivalent to 50 mg of dexketoprofen.
- -The other ingredients are: sodium chloride, ethanol 96%, sodium hydroxide (for pH adjustment), water for injections.
What Auxilen looks like and contents of the packaging
Clear, colorless solution, free from visible particles.
Auxilen is produced in ampoules made of colored glass type I, each containing 2 mL.
The packaging contains: 1, 5, 6, 10, 25, or 100 ampoules.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
AS KALCEKS
Krustpils iela 71E
1057 Rīga
Latvia
Phone: +371 67083320
Email: [email protected]
Date of last revision of the leaflet:
This medicine is authorized in the Member States of the European Economic Area under the following names:
Estonia
Dexketoprofen Kalceks
Latvia
Dexketoprofen Kalceks 50 mg/2 ml šķīdums injekcijām/infūzijām
Lithuania
Dexketoprofen Kalceks 50 mg/2 ml injekcinis ar infuzinis tirpalas
Romania
Xedofen 50 mg/2 ml soluţie injectabilă/perfuzabilă
Bulgaria
Auxilen 50 mg/2 ml инжекционен/инфузионен разтвор
Ireland
Morsadex 50 mg/2 ml solution for injection/infusion
Poland
Auxilen
Austria
Auxilen 50 mg/2 ml Injektions-/Infusionslösung
Germany
Dexketoprofen Ethypharm Kalceks 50 mg Injektions-/Infusionslösung
Spain
Auxilen 50 mg/2 ml solución inyectable y para perfusión EFG
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Intravenous administration:
Intravenous infusion:dilute the contents of 1 ampoule (2 mL) of Auxilen in a volume of 30 mL to 100 mL of 0.9% sodium chloride solution, 5% glucose solution, or Ringer's lactate solution.
The diluted solution should be administered as a slow intravenous infusion over a period of 10 minutes to 30 minutes. The solution should always be protected from daylight.
Intravenous bolus (intravenous injection):if necessary, the contents of 1 ampoule (2 mL) of Auxilen can be administered as a slow intravenous bolus over a period of not less than 15 seconds.
Due to the ethanol content, Auxilen should not be administered directly into the spinal canal (intrathecally or epidurally).
Instructions for handling the medicine:
When administering Auxilen as an intravenous bolus, the solution should be injected immediately after withdrawal from the colored ampoule.
When administering as an intravenous infusion, the solution should be diluted under aseptic conditions and protected from daylight.
Only clear and colorless solutions should be used.
Compatibility information:
It has been shown that Auxilen is compatible when mixed
The diluted solution is a clear solution. Auxilen diluted
No absorption of the active substance was observed when diluted solutions of Auxilen were stored in plastic bags or devices for administration made of ethyl vinyl acetate (EVA), cellulose propionate (CP), low-density polyethylene (LDPE), and polyvinyl chloride (PVC).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAS Kalceks
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AuxilenDosage form: Solution, 50 mg/2 mlActive substance: dexketoprofenPrescription requiredDosage form: Tablets, 25 mgActive substance: dexketoprofenPrescription not requiredDosage form: Tablets, 25 mgActive substance: dexketoprofenManufacturer: Galenicum Health, S.L.U. SAG Manufacturing S.L.U.Prescription not required
Alternatives to Auxilen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Auxilen in Espanha
Alternative to Auxilen in Ukraine
Online doctors for Auxilen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Auxilen – subject to medical assessment and local rules.














