
Atracurium Kalceks

Ask a doctor about a prescription for Atracurium Kalceks

How to use Atracurium Kalceks
Leaflet accompanying the packaging: patient information
Atracurium Kalceks, 10 mg/ml, solution for injection/infusion
Atracurium besilate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Atracurium Kalceks and what is it used for
- 2. Important information before using Atracurium Kalceks
- 3. How to use Atracurium Kalceks
- 4. Possible side effects
- 5. How to store Atracurium Kalceks
- 6. Contents of the packaging and other information
1. What is Atracurium Kalceks and what is it used for
Atracurium Kalceks belongs to a group of medicines commonly known as skeletal muscle relaxants.
Atracurium Kalceks is used during surgical operations to relax muscles, allow the insertion of an endotracheal tube, and facilitate controlled ventilation. It is also used to facilitate mechanical ventilation in patients in intensive care units.
2. Important information before using Atracurium Kalceks
When not to use Atracurium Kalceks:
- if the patient is allergic to atracurium besilate, cisatracurium or any of the other ingredients of this medicine (listed in section 6).
If this applies to the patient, tell the doctor before administering Atracurium Kalceks.
Warnings and precautions
Before starting treatment with Atracurium Kalceks, discuss it with your doctor or nurse:
- if the patient has an allergy or asthma;
- if the patient has ever had an allergic reaction to other medicines similar to Atracurium Kalceks that block the transmission of impulses between nerves and muscles;
- if the patient is weakened, has muscle fatigue or impaired movement coordination (myasthenia gravis);
- if the patient has a neuromuscular disease;
- if the patient has heart disease or is sensitive to blood pressure drops;
- if the patient has electrolyte disturbances (abnormal levels of ions such as sodium, potassium or chloride in the blood);
- if the patient has recently had severe burns requiring medical attention.
If any of the above points apply to the patient, tell the doctor.
Children
This medicine is not intended for children under 1 month of age.
Atracurium Kalceks and other medicines
Tell your doctor about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
Some medicines may affect the action of Atracurium Kalceks. Tell your doctor if the patient is taking any of the following medicines:
- anesthetics (used to reduce consciousness and pain during surgery), such as halothane, isoflurane, enflurane or ketamine;
- antibiotics (used to treat infections), such as aminoglycosides, polymyxins, spectinomycin, tetracyclines, lincomycin and clindamycin;
- antiarrhythmic medicines (used to treat heart rhythm disorders), such as propranolol, oxprenolol, calcium channel blockers, lidocaine, procainamide and quinidine;
- diuretics (used to treat high blood pressure), such as furosemide, mannitol, thiazide diuretics and acetazolamide;
- magnesium salts (used to prevent magnesium deficiency);
- medicines used to treat mental disorders, such as lithium or chlorpromazine;
- medicines used to treat high blood pressure (hypertension), such as trimethaphan, hexamethonium;
- medicines used to treat joint inflammation (anti-rheumatic medicines), such as chloroquine and penicillamine;
- medicines used to treat Alzheimer's disease, such as donepezil;
- steroids (used to treat inflammation or asthma), such as prednisolone;
- medicines used to treat epilepsy (seizures), such as phenytoin.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant or plans to have a child, she should consult her doctor before using this medicine. The doctor will weigh the benefits and risks before administering Atracurium Kalceks.
Atracurium Kalceks can be used to maintain muscle relaxation during cesarean section.
Driving and using machines
Atracurium Kalceks has a significant impact on the ability to drive and use machines. Consult your doctor when you can safely resume driving and using machines.
Do not drive or operate machines if you feel unwell.
3. How to use Atracurium Kalceks
Atracurium Kalceks is used during procedures where the patient is anesthetized (unconscious) or heavily sedated. This medicine will always be administered under the supervision of an experienced doctor.
What dose is given
The doctor will determine the correct dose of Atracurium Kalceks based on:
- the patient's body weight;
- the required range and duration of muscle relaxation;
- the expected response (reaction) of the patient to the administration of the medicine.
How Atracurium Kalceks is given
Atracurium Kalceks is given as a single injection into a vein or as an intravenous infusion (usually using an infusion pump). In this case, the medicine is administered slowly over a set period of time.
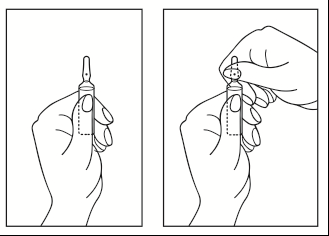
Instructions for opening the ampoule:
- 1) Position the ampoule so that the colored dot is at the top. If there is a portion of the solution at the top of the ampoule, gently tap with your finger to ensure the entire solution is in the lower part of the ampoule.
- 2) Use both hands to open; holding the lower part of the ampoule in one hand, break off the top part of the ampoule in the opposite direction of the colored dot (see pictures below).
Use in children
This medicine should not be used in children under 1 month of age.
Overdose of Atracurium Kalceks
This medicine is given only by properly trained doctors. Since the medicine is administered to the patient during hospitalization, it is unlikely that the patient will receive too little or too much medicine. However, if the patient has any concerns, they should immediately inform their doctor or medical staff. In case of an overdose, appropriate action will be taken immediately.
4. Possible side effects
Like all medicines, Atracurium Kalceks can cause side effects, although not everybody gets them.
If the patient experiences any of the following side effects, they should tell their doctor immediately:
- severe allergic reaction - the patient may experience sudden, itchy rash, swelling of the hands, feet, ankles, face, lips, tongue or throat (which may cause difficulty swallowing or breathing), the patient may feel faint;
- anaphylactic shock;
- heart failure;
- cardiac arrest.
The above side effects are very rare. The patient may need immediate medical attention.
If the patient experiences any of the following side effects, they should tell their doctor as soon as possible:
Common side effects(may affect up to 1 in 10 people)
- low blood pressure (hypotension), usually mild and transient;
- redness of the skin.
Uncommon side effects(may affect up to 1 in 100 people):
- breathing difficulties and wheezing (bronchospasm).
Side effects with unknown frequency(frequency cannot be estimated from available data):
- seizures;
- muscle disorders (myopathy), muscle weakness.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, PL-02 222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Atracurium Kalceks
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the ampoule label after: "EXP" and on the carton after: "Expiry date (EXP)". The expiry date refers to the last day of the month.
Store in a refrigerator (2°C – 8°C). Do not freeze.
Store in the original packaging to protect from light.
For single use only. After opening, the medicine should be used immediately.
Before use, the solution should be inspected carefully. Only clear solutions without visible particles should be used.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Atracurium Kalceks contains
- The active substance is atracurium besilate. 1 ml of solution contains 10 mg of atracurium besilate. One ampoule (2.5 ml) contains 25 mg of atracurium besilate. One ampoule (5 ml) contains 50 mg of atracurium besilate.
- The other ingredients are: benzenesulfonic acid (for pH adjustment), water for injections.
What Atracurium Kalceks looks like and contents of the pack
Clear, colorless to yellowish solution, without visible particles.
2.5 ml or 5.0 ml solution in 5.0 ml ampoules made of colorless glass (type I).
The ampoules are packed in a PVC sleeve. The sleeve is packed in a cardboard box.
Pack sizes: 1 or 5 ampoules.
Not all pack sizes may be marketed.
Marketing authorization holder
AS KALCEKS
Krustpils iela 71E
1057 Rīga
Latvia
Tel.: +371 67083320
Email: [email protected]
Manufacturer
Akciju sabiedrība “Kalceks”
Krustpils iela 71E
1057 Rīga
Latvia
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom under the following names:
Bulgaria
Atracurium Kalceks 10 mg/ml инжекционен/инфузионен разтвор
Belgium
Atracurium Kalceks 10 mg/ml solution injectable/pour perfusion
Atracurium Kalceks 10 mg/ml oplossing voor injectie/infusie
Atracurium Kalceks 10 mg/ml Injektions-/Infusionslösung
Czech Republic
Atracurium Kalceks
Estonia
Atracurium besilate Kalceks
France
ATRACURIUM KALCEKS 10 mg/ml, solution injectable/pour perfusion
Hungary
Atracurium besilate Kalceks 10 mg/ml oldatos injekció/infúzió
Ireland
Atracurium besilate 10 mg/ml solution for injection/infusion
Latvia
Atracurium besilate Kalceks 10 mg/ml šķīdums injekcijām/infūzijām
Lithuania
Atracurium besilate Kalceks 10 mg/ml injekcinis ar infuzinis tirpalas
Malta
Atracurium Kalceks 10 mg/ml solution for injection/infusion
Netherlands
Atracurium Kalceks 10 mg/ml oplossing voor injectie/infusie
Poland
Atracurium Kalceks
Romania
Atracurium Kalceks 10 mg/ml soluție injectabilă/perfuzabilă
Slovakia
Atracurium Kalceks 10 mg/ml injekčný/infúzny roztok
Spain
Besilato de Atracurio Kalceks 10 mg/ml soluciόn inyectable y para perfusiόn
Sweden
Atracurium Kalceks 10 mg/ml injektions-/infusionsvätska, lösning
United Kingdom (Northern Ireland)
Atracurium besilate 10 mg/ml solution for injection/infusion
Date of last revision of the leaflet: ------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
For single use only. After opening, the medicine should be used immediately.
From a microbiological point of view, the medicine should be used immediately, unless the method of dilution precludes the risk of microbial contamination. If not used immediately, the user is responsible for the storage conditions and time.
Atracurium besilate is inactivated at high pH and therefore should not be mixed in the same syringe with thiopental or other alkaline medicines.
In the case of choosing a vein with a small diameter as the site of administration, after injecting Atracurium Kalceks, the vein should be flushed with an isotonic sodium chloride solution. When Atracurium Kalceks and other anesthetics are administered through a permanently inserted needle or cannula, after injecting each medicine, the vein should be flushed with an appropriate volume of isotonic sodium chloride solution.
Since Atracurium Kalceks is a hypotonic compound, it should not be administered through a cannula intended for blood transfusion.
Atracurium Kalceks can be diluted with the following infusion solutions.
| Infusion solution | Stability |
| Sodium chloride injection solution (9 mg/ml) | 24 hours |
| Glucose injection solution (50 mg/ml) | 8 hours |
| Ringer's solution for intravenous injection | 8 hours |
| Sodium chloride (1.8 mg/ml) and glucose (40 mg/ml) injection solution | 8 hours |
| Ringer's solution with lactate | 4 hours |
After dilution with one of the above solutions to a concentration of atracurium besilate of 0.5 mg/ml or more, the resulting solutions are stable in daylight for the periods indicated above, provided they are stored at a temperature below 25°C.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAkciju sabiedriba "Kalceks"
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Atracurium KalceksDosage form: Solution, 10 mg/mlActive substance: atracuriumManufacturer: Aspen Bad Oldesloe GmbH Aspen Pharma Ireland Limited GlaxoSmithKline Manufacturing S.p.A.Prescription not requiredDosage form: Powder, 4 mgActive substance: pipecuronium bromideManufacturer: Gedeon Richter Plc.Prescription not requiredDosage form: Solution, 5 mg/mlActive substance: cisatracuriumPrescription not required
Alternatives to Atracurium Kalceks in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Atracurium Kalceks in Испания
Alternative to Atracurium Kalceks in Украина
Online doctors for Atracurium Kalceks
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Atracurium Kalceks – subject to medical assessment and local rules.














