
Asmanex Tvisthaler

Ask a doctor about a prescription for Asmanex Tvisthaler

How to use Asmanex Tvisthaler
Leaflet accompanying the packaging: patient information
Asmanex Twisthaler, 200 micrograms/dose, inhalation powder
Mometasone furoate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What Asmanex Twisthaler is and what it is used for
- 2. Important information before using Asmanex Twisthaler
- 3. How to use Asmanex Twisthaler
- 4. Possible side effects
- 5. How to store Asmanex Twisthaler
- 6. Contents of the packaging and other information
1. What Asmanex Twisthaler is and what it is used for
Asmanex Twisthaler contains the active substance mometasone furoate, which belongs to a group of medicines called corticosteroids or steroids.
Do not confuse corticosteroids with anabolic steroids, which are misused by some athletes. Corticosteroids are used to prevent asthma attacks due to their anti-inflammatory effect. They reduce swelling and irritation of the airway walls, making it easier to breathe.
Regular use of Asmanex Twisthaler prevents asthma symptoms in adults and adolescents aged 12 and over. The medicine can be used in patients who have not previously been treated with steroids, or in those whose asthma is not controlled by other medicines. It can also be given to patients who are taking other steroids, oral or inhaled, for asthma treatment.
Regular use of Asmanex Twisthaler helps control asthma symptoms and prevents sudden asthma attacks. The medicine is used preventively. It should not be used to stop a sudden asthma attack. You should always carry a prescribed bronchodilator (inhaler that makes breathing easier) with you in case of an asthma attack.
2. Important information before using Asmanex Twisthaler
When not to use Asmanex Twisthaler
Warnings and precautions
Before starting treatment with Asmanex Twisthaler, discuss with your doctor or pharmacist if:
- the patient currently has or has had tuberculosis in the past,
- the patient has a viral eye infection (such as herpes) or any other type of infection,
- the patient has thrush (white patches in the mouth and throat), as appropriate treatment may be necessary. The doctor may recommend temporary discontinuation of Asmanex Twisthaler or treatment with another inhaled medicine. After taking the medicine, rinse your mouth with water or mouthwash and spit it out, do not swallow. This may help prevent thrush.
- If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Important information to remember while using Asmanex Twisthaler
- In case of sudden wheezing and difficulty breathing shortly after taking Asmanex Twisthaler, call emergency services or go to the emergency department of the nearest hospital. These symptoms may indicate an allergic reaction to the medicine.
- Always carry a reliever inhaler (inhaler that makes breathing easier) with you to quickly relieve bronchospasm or asthma attacks (coughing, wheezing, and chest tightness).
- If an asthma attack occurs that does not improve with the use of a reliever inhaler (inhaler that makes breathing easier), or if the peak expiratory flow rate decreases, call emergency services or go to the emergency department of the nearest hospital. Inform your doctor, as this may indicate worsening asthma and a change in medication may be necessary.
- In case of a severe asthma attack, other illnesses, or stress, it may be necessary to take a steroid in tablet or syrup form. Your doctor may recommend that you always carry a certain amount of steroids in tablet or syrup form and an information card with dosage instructions and guidelines on when to use the oral steroid.
- If you are taking Asmanex Twisthaler and reducing the dose of steroids in tablet or syrup form, you may experience allergic reactions that have not occurred before, such as itching, excessive tearing, or rash. In this case, your doctor will recommend appropriate action to control these symptoms. If you experience joint or muscle pain, feelings of depression, fatigue, or significant slowing down during this period, inform your doctor.
- While using Asmanex Twisthaler, avoid contact with people who have chickenpox or shingles. If you come into contact with someone who has one of these illnesses, inform your doctor. This is especially important for children.
- The use of medicines like Asmanex Twisthaler may disrupt the normal production of steroids in the body. As a result, long-term treatment in adolescents may lead to growth retardation. Your doctor will likely monitor your growth periodically.
- All steroids, especially when used for a long time, may disrupt the function of the adrenal gland. Therefore, your doctor will regularly perform blood tests to monitor adrenal function.
- If you are hospitalized, take Asmanex Twisthaler and other medicines you are taking with you.
- Your doctor may perform lung function tests while you are using Asmanex Twisthaler, especially if you are going to stop taking oral corticosteroids.
Children and adolescents
Due to the lack of available data, Asmanex Twisthaler should not be used in children under 12 years of age.
Asmanex Twisthaler and other medicines
Tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, including those that are available without a prescription, as well as any medicines you plan to take, including those used to treat fungal or viral infections, such as ketoconazole, itraconazole, ritonavir, nelfinavir, or cobicistat. These medicines may increase the levels of mometasone furoate in your blood.
Some medicines may enhance the effect of Asmanex Twisthaler, and your doctor may want to monitor your condition closely while you are taking such medicines (including certain HIV medicines: ritonavir, cobicistat).
It is especially important to inform your doctor before starting treatment with Asmanex Twisthaler if you are taking corticosteroids, either by injection, orally, or inhaled. Your doctor will adjust the dose of these corticosteroids.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine.
Do not use Asmanex Twisthaler in pregnant women unless your doctor explicitly recommends it.
There is no data on the passage of the medicine into breast milk. Do not breastfeed while using this medicine unless your doctor explicitly recommends it.
Driving and using machines
Asmanex Twisthaler has no or negligible influence on the ability to drive and use machines.
Asmanex Twisthaler contains lactose
The medicine contains small amounts of lactose (the maximum recommended daily dose contains 4.64 mg of lactose). This amount of lactose usually does not cause problems in patients with lactose intolerance.
3. How to use Asmanex Twisthaler
The patient must be sure they can use the Twisthaler inhaler correctly. If in doubt, consult your doctor or pharmacist. Before using the medicine, the patient must be sure when and how many doses to take. The doctor should inform the patient how to use Asmanex Twisthaler. Read the instructions for using the inhaler. This medicine should always be used as recommended by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist.
Do not change the dose of the medicine without your doctor's explicit recommendation.
In adults (including those over 65) and adolescents aged 12 and over with mild or moderate asthma, the recommended initial dose is 400 micrograms (2 inhalations) once daily, in the evening.
In some patients, the initial dose may be 200 micrograms (1 inhalation) twice daily. If improvement occurs, the doctor may reduce the dose to 200 micrograms (1 inhalation) once daily, in the evening. Depending on the symptoms, the doctor may increase or decrease the daily dose to keep asthma symptoms under control.
In patients with severe asthma who may also be taking other steroids in tablet or syrup form, the usual initial dose of Asmanex Twisthaler is 400 micrograms twice daily. This is the maximum recommended dose. After about one week of using Asmanex Twisthaler, the doctor may recommend gradually reducing the dose of the other corticosteroid until it is completely stopped. If asthma symptoms are well-controlled, it may also be possible to reduce the dose of Asmanex Twisthaler.
Instructions for proper use of the inhaler
Remember that the fine powder released from the Twisthaler inhaler must be inhaled into the lungs. Stand upright while using the Twisthaler inhaler. Follow the instructions below carefully to take the correct dose.
Before removing the white cap, make sure the counter and the arrow on the cap are in line.
To open the inhaler, remove the white cap. Hold the inhaler upright with the pink base down. Grasp the base and twist the cap counterclockwise. After removing the cap, the dose counter on the inhaler will decrease by one.
Make sure the counter on the pink base and the arrow on the upper counter are in line. When the cap is removed and before taking the correct dose, hold the inhaler upright.
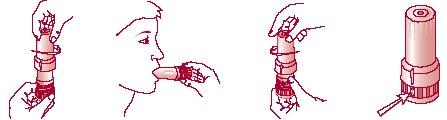
To inhale the dose, follow these steps:
a)
Remove the cap from the inhaler (Figure 1).
b)
Bring the inhaler to your mouth, with the mouthpiece facing you.
c)
Put the mouthpiece in your mouth, close your lips around it, and take a quick, deep breath (Figure 2).
d)
Remove the inhaler from your mouth and hold your breath for about 10 seconds or as long as possible. Do not breathe out through the inhaler.
e)
To close the inhaler, replace the cap immediately after each inhalation.
To load a new dose, put the cap back on and twist it clockwise while gently pressing it until you hear a "click," which means the cap is fully closed (Figure 3). The arrow on the cap must be fully in line with the counter window (Figure 4).
After taking the medicine, rinse your mouth with water or mouthwash and spit it out, do not swallow. This may help prevent thrush.
The inhaler should always be kept dry and clean. Clean the mouthpiece surface with a dry cloth or tissue. Do not wash the inhaler, and avoid contact with water.
The base of the inhaler has a window with a dose counter that shows the number of doses (inhalations) left in the inhaler. Do not use Asmanex Twisthaler if the counter readings are not correct. In this case, consult your doctor or pharmacist.
If the counter window shows "01," it means there is one dose left in the inhaler. After the "01" dose, the counter will show "00," the cap will be locked, and it will not be possible to take further doses from this inhaler. Dispose of it.
Before using the used packaging, obtain a new prescription from your doctor to ensure you can always use the medicine.
The time it takes to see an improvement varies. In some patients, improvement occurs within 24 hours of starting treatment, while in others it may take 1 or 2 weeks, or even longer, before satisfactory improvement is seen. The duration of treatment with Asmanex Twisthaler will be decided by your doctor.
Using a higher dose of Asmanex Twisthaler than recommended
It is very important to use the medicine as recommended by your doctor. Do not increase or decrease the dose of the medicine without your doctor's recommendation. Consult your doctor if you have accidentally taken a higher dose of the medicine than recommended.
Missing a dose of Asmanex Twisthaler
If you miss a dose, take the next dose at the usual time. Do not take a double dose to make up for the missed dose.
Stopping treatment with Asmanex Twisthaler
Do not suddenly stop using Asmanex Twisthaler, even if your symptoms improve.
Consult your doctor first.
Relapse of symptoms may occur if you stop treatment without consulting your doctor.
If you find that your symptoms do not improve or worsen after starting treatment with Asmanex Twisthaler, consult your doctor.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Asmanex Twisthaler can cause side effects, although not everybody gets them.
Stop using Asmanex Twisthaler and contact your doctor immediately if you experience any of the following symptoms:
symptoms of a severe allergic reaction, including swelling of the eyes, face, lips, tongue, or throat, which may cause difficulty swallowing or breathing, itching rash, dizziness, and fainting, which may cause collapse.
If you experience worsening symptoms, such as coughing, wheezing, difficulty breathing, or shortness of breath, after inhaling the medicine, use your reliever inhaler and contact your doctor immediately. Do not use Asmanex Twisthaler again until you have consulted your doctor.
An allergic reaction (hypersensitivity) to inhaled steroids may occur. Symptoms include rash, itching, and redness and swelling of the eyes, face, lips, and throat. If you experience any of these symptoms, contact your doctor immediately. Do not use Asmanex Twisthaler again until you have consulted your doctor.
The most common side effects include fungal infections (thrush) of the mouth or throat (white patches appear) (very common, i.e., more than 1 in 10 people using the medicine, in the group of patients receiving 400 micrograms of mometasone furoate twice daily), hoarseness, sore throat, or headache (common, i.e., not more than 1 in 10 people using the medicine, in the group of patients receiving 400 micrograms of mometasone furoate twice daily). Contact your doctor as soon as possible if you experience any of these side effects.
Other side effects that occur not very commonly (not more than 1 in 100 people using the medicine) include dryness in the mouth and throat, indigestion, weight gain, and rapid or strong heartbeat (palpitations). Contact your doctor if you experience any of these symptoms.
In patients using inhaled steroids, including Asmanex Twisthaler, rare (not more than 1 in 1,000 people using the medicine) cases of increased eye pressure (including glaucoma) or cataracts may occur.
Frequency not known (cannot be estimated from the available data): blurred vision.
Contact your doctor if you experience blurred vision or eye pain.
Other side effects that may occur when using steroids include growth retardation or changes in growth rate in adolescents, as well as decreased bone mineral density.
When using inhaled corticosteroids, sleep disturbances, depression, feelings of anxiety, fear, nervousness, excessive excitement, or irritability (frequency not known) may also occur. The occurrence of these side effects is more likely in children.
Steroids may also disrupt adrenal function, causing weakness, fatigue, dizziness, and fainting when standing for a long time or changing position from sitting to standing.
The occurrence of these side effects is much less likely when using inhaled corticosteroids than when using oral corticosteroids.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Asmanex Twisthaler
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging, foil bag, and label after: EXP. The expiry date refers to the last day of the month stated.
Store in a temperature below 30°C. Do not store in the refrigerator or freeze.
Store in the original packaging to protect from moisture. The medicine remains stable for 2 months after first opening. After this time, the medicine should be disposed of in accordance with local regulations.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Asmanex Twisthaler contains
- The active substance of the medicine is mometasone furoate micronized. One dose of Asmanex Twisthaler contains 200 micrograms of mometasone furoate micronized.
- The other ingredient is lactose anhydrous.
What Asmanex Twisthaler looks like and what the packaging contains
The inhaler has a white mouthpiece and a pink base containing a dose counter window that shows the number of doses (inhalations) left in the inhaler.
The inhaler contains 30 or 60 doses. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Organon Polska Sp. z o.o.
Marszałkowska 126/134
00-008 Warsaw
Tel.: +48 22 105 50 01
[email protected]
Manufacturer
Organon Heist bv
Industriepark 30
2220 Heist-op-den-Berg
Belgium
Date of last revision of the leaflet: 11/2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOrganon Heist bv
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Asmanex TvisthalerDosage form: Powder, 400 mcg/dose inh.Active substance: mometasoneManufacturer: Organon Heist bvPrescription requiredDosage form: Aerosol, 160 mcg/dose inh.Active substance: ciclesonidePrescription requiredDosage form: Aerosol, 160 mcg/dose inh.Active substance: ciclesonidePrescription required
Alternatives to Asmanex Tvisthaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Asmanex Tvisthaler in Spain
Online doctors for Asmanex Tvisthaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Asmanex Tvisthaler – subject to medical assessment and local rules.














