
Artelac

Ask a doctor about a prescription for Artelac

How to use Artelac
Package Leaflet: Information for the User
Artelac
3.2 mg/ml, eye drops, solution
(Hypromellose)
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- If there is no improvement or the patient feels worse, they should contact their doctor.
Table of Contents of the Leaflet:
- 1. What is Artelac and what is it used for
- 2. Important information before using Artelac
- 3. How to use Artelac
- 4. Possible side effects
- 5. How to store Artelac
- 6. Contents of the pack and other information
1. What is Artelac and what is it used for
Artelac is an eye drop solution. The active substance is hypromellose.
Indications
Artelac is used for the symptomatic treatment of dry eye syndrome. Dry eye syndrome may occur when there is a deficiency in tear secretion or when the eyelids cannot be fully or partially closed.
The medicine may also be used to moisten hard contact lenses.
If there is no improvement or the patient feels worse, they should contact their doctor.
One in five patients treated by an ophthalmologist has symptoms of dry eye syndrome. There are many causes of this condition, such as: decreased tear production in older age, working in an air-conditioned room, working on a computer, hormonal changes during menopause, etc. Environmental pollution and climate can also play a role in the development of dry eye syndrome.
Understanding the term "dry eye"
During the day, we blink our eyelids about 14,000 times on average. With each blink, a very thin layer of tears is spread over the surface of the eyeball, which maintains smoothness, cleanliness, and moisture, and protects against environmental pollutants. If the amount or composition of the tear fluid is disturbed, the phenomenon of drying of the cornea and conjunctiva occurs, causing symptoms such as: burning, feeling of dry eye, feeling of sand in the eye, feeling of pressure, and sensitivity to light.
2. Important information before using Artelac
When not to use Artelac
Warnings and precautions
Before starting to use Artelac, the patient should discuss it with their doctor or pharmacist:
- Artelac is intended for use in the eyes only.
- If symptoms of eye irritation, eye pain, redness of the eyes, vision problems occur or worsen, the patient should stop using the medicine and consult an ophthalmologist.
- If the symptoms of dry eye syndrome persist or worsen, the patient should consult their doctor.
- The patient should remove their contact lenses before administering the medicine and not put them back in for at least 15 minutes.
Artelac and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Interactions with other medicines are not known.
If Artelac is used with other locally administered eye medicines, the patient should wait 15 minutes between administrations. Artelac should always be administered last, after 15 minutes from the administration of another medicine, to ensure sufficient time for the medicine to work and have a moisturizing effect.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Artelac should not be used during pregnancy and breastfeeding, unless the doctor decides otherwise.
Driving and using machines
Artelac may temporarily blur vision after administration. The patient should not drive or operate machinery until their vision is clear.
Artelac contains cetrimide as a preservative. It can cause eye irritation (burning, redness, feeling of a foreign body) and damage to the corneal epithelium, especially when used for a long time.
Therefore, for long-term or continuous treatment of dry eye syndrome, preservative-free medicines are recommended.
Artelac contains disodium dihydrogen phosphate dihydrate and disodium hydrogen phosphate dodecahydrate
(1 ml of solution contains 1.84 mg of phosphates).
In patients with severe damage to the transparent front part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding due to calcium deposition during treatment.
3. How to use Artelac
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
Recommended dose
Treatment of dry eye syndrome requires individual dosing.
Depending on the severity and intensity of the symptoms, usually one drop is instilled into the conjunctival sac 3 to 5 times a day, or more often, as needed.
The patient should consult their ophthalmologist if they need long-term or continuous treatment with Artelac.
The medicine can also be used to moisten hard contact lenses.
Instructions for administering the medicine:
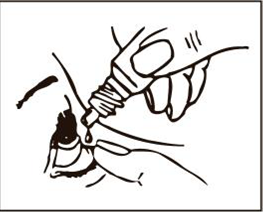
Caution!
To avoid contaminating the eye drops, do not touch the dropper tip with your fingers or touch it to the surface of the eye or any other surface.
Using contaminated drops can lead to serious eye damage, including vision loss.
- 1. Wash your hands thoroughly.
- 2. Remove the protective cap.
- 3. Hold the bottle vertically over the eye, holding it between the thumb and index finger.
- 4. Tilt your head back.
- 5. With the index finger of the other hand, gently pull down the lower eyelid to create a "pocket" between the eyeball and the eyelid, into which the medicine will be instilled.
- 6. Bring the tip of the bottle close to the eye, without touching the dropper to the eye, eyelid, or surrounding areas.
- 7. Gently squeeze the bottle to release a single drop of medicine.
- 8. Looking up, put 1 drop of medicine into the created "pocket". If the drop does not get into the eye, repeat the action.
- 9. Try to keep the eye open and move it to distribute the drop evenly.
- 10. If drops are to be administered to both eyes, repeat the above steps for the other eye.
- 11. Close the bottle immediately after use.
Having someone else help or using a mirror can make it easier to administer the medicine.
If the patient feels that the effect of Artelac is too strong or too weak, they should consult their doctor or pharmacist.
Using a higher dose of Artelac than recommended
If a higher dose of the medicine is used than recommended, it may cause vision disturbances, which will quickly resolve.
Missing a dose of Artelac
If a dose is missed, the next dose should be used at the usual time, according to the recommended dosing schedule. The patient should not use a double dose to make up for the missed dose.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Artelac can cause side effects, although not everybody gets them.
The frequency of possible side effects is defined as follows:
Very common -
occurring in more than 1 in 10 patients
Common -
occurring in 1 to 10 in 100 patients
Uncommon -
occurring in 1 to 10 in 1,000 patients
Rare -
occurring in 1 to 10 in 10,000 patients
Very rare -
occurring in less than 1 in 10,000 patients
Frequency not known - cannot be estimated from the available data
The following side effects may occur:
Frequency not known:
- Conjunctival hyperemia
- Eye irritation
- Eye pain
- Eye itching
- Eye burning
- Eye redness
- Feeling of a foreign body in the eye
- Excessive tearing
- Eye lid sticking together
- Vision disturbances
- Hypersensitivity
- Itching
- Rash
- Corneal clouding due to calcium deposition (see section 2 for more information)
Reporting side effects
If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Pharmacovigilance, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Artelac
The medicine should be kept out of the sight and reach of children.
The medicine should be stored at a temperature below 25°C.
Shelf life after first opening the bottle – 6 weeks.
Any unused contents of the pack should be discarded 6 weeks after first opening the bottle.
Do not use this medicine after the expiry date stated on the outer packaging or on the blister/foil, or after the "EXP" date. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Artelac contains
- The active substance is hypromellose. 1 ml of solution contains 3.2 mg of hypromellose
- The other ingredients are: cetrimide, disodium edetate, disodium dihydrogen phosphate dihydrate, disodium hydrogen phosphate dodecahydrate, sorbitol, water for injections.
What Artelac looks like and contents of the pack
Artelac is an eye drop solution.
Pack sizes:
Bottle containing 10 ml of eye drops
Marketing authorization holder and manufacturer
Dr. Gerhard Mann
Chem.-Pharm. Fabrik GmbH
Brunsbütteler Damm 165/173
- D - 13581 Berlin Germany
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterDr. Gerhard Mann Chem. - Pharm. Fabrik GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ArtelacDosage form: Drops, 3.2 mg/mlActive substance: hypromellosePrescription not requiredDosage form: Drops, 3.2 mg/mlActive substance: hypromellosePrescription not requiredDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not required
Online doctors for Artelac
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Artelac – subject to medical assessment and local rules.














