
Aminoplasmal Paed 10%

Ask a doctor about a prescription for Aminoplasmal Paed 10%

How to use Aminoplasmal Paed 10%
Leaflet accompanying the packaging: information for the user
Aminoplasmal Paed 10%, solution for infusion
For use in children (aged 0-11 years)
Amino acids
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or nurse.
- This medicine has been prescribed specifically for this child. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as the child's.
- If the child experiences any side effects, including any not listed in this leaflet, tell the doctor or nurse. See section 4.
Table of contents of the leaflet
- 1. What Aminoplasmal Paed 10% is and what it is used for
- 2. Important information before using Aminoplasmal Paed 10% in children
- 3. How to use Aminoplasmal Paed 10%
- 4. Possible side effects
- 5. How to store Aminoplasmal Paed 10%
- 6. Contents of the packaging and other information
1. What Aminoplasmal Paed 10% is and what it is used for
Aminoplasmal Paed 10% is a solution administered to the patient through a small tube with a needle inserted into a vein (intravenous infusion).
The solution contains amino acids, which are essential for the body's growth or recovery.
The solution has been prepared to meet the specific needs of premature infants, full-term newborns, and small and older children.
This medicine is given to them if they are unable to take food normally or cannot be fed through a tube inserted into the stomach. At the same time, they can also take other nutritional components, such as glucose solutions or fat emulsions, with Aminoplasmal Paed 10%.
2. Important information before using Aminoplasmal Paed 10% in children
When not to use Aminoplasmal Paed 10%
- if the child is allergic to the active substances or any of the other ingredients of this medicine (listed in section 6);
- if the child has a congenital defect in protein and amino acid metabolism;
- if the child has severe (life-threatening) circulatory disorders (shock);
- if the child has insufficient oxygen supply (hypoxia);
- if acidic substances have accumulated in the child's blood (metabolic acidosis);
- if the child has severe liver disease (severe liver failure);
- if the child has severe kidney failure and is not properly treated with artificial kidney or similar therapy;
- if the child has insufficiently controlled heart failure with significant circulatory disorders (uncompensated heart failure);
- if fluid has accumulated in the child's lungs (pulmonary edema);
- if the child has disorders of fluid or mineral balance (electrolyte imbalance).
Warnings and precautions
When used in children from premature to 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration.
Exposure of parenteral nutrition solutions containing Aminoplasmal Paed 10% to environmental light, especially after adding trace elements and/or vitamins, leads to the formation of peroxides and other degradation products, which can be limited by protecting against light.
Before starting Aminoplasmal Paed 10%, discuss with the doctor or pharmacist if the child:
- has protein and amino acid metabolism disorders for reasons other than those listed above (see "When not to use Aminoplasmal Paed 10%...");
- has liver or kidney function disorders;
- has heart function disorders;
- has highly concentrated blood serum (high osmolality of serum).
In case of fluid or mineral balance disorders, such disorders should be corrected before administering this medicine. Examples of such disorders include concurrent fluid and mineral deficiencies (hypotonic dehydration), sodium deficiency (hyponatremia), or potassium deficiency (hypokalemia).
Before and during administration of this solution to children, the doctor will monitor the levels of minerals in the blood, blood sugar levels, fluid balance, and acid-base balance. The levels of proteins in the blood and liver and kidney function will also be monitored. To this end, blood and urine samples will be taken and analyzed.
Amino acid solutions are only one component of parenteral nutrition. Children usually receive Aminoplasmal Paed 10% as part of intravenous nutrition, which also includes non-protein energy supplements (carbohydrate solutions, fat emulsions), essential fatty acids, electrolytes, vitamins, fluids, and trace elements.
Aminoplasmal Paed 10% and other medicines
Tell the doctor or nurse about all medicines the child is taking or has recently taken, as well as any medicines the child plans to take.
Pregnancy and breastfeeding
Aminoplasmal Paed 10% is intended for use only in children (under 12 years of age).
Driving and using machines
Not applicable.
3. How to use Aminoplasmal Paed 10%
Aminoplasmal Paed 10% is administered by medical personnel.
The doctor will carefully adjust the dose based on the child's age, development stage, and underlying disease.
The administered doses will be approximately:
Premature newborns:
40 ml per kg of body weight per day
Full-term newborns (aged 0 to 27 days):
30 ml per kg of body weight per day
Infants and small children (aged 28 days to 23 months):
25 ml per kg of body weight per day
Older children (aged 2 to 11 years):
20 ml per kg of body weight per day
In the case of children in severe condition, the administered dose may be higher (up to 30 ml per kg of body weight per day).
Patients with liver or kidney disease
If the child has liver or kidney disease, the doses will be adjusted according to their individual needs.
Duration of treatment
This medicine can be used for as long as the child requires parenteral nutrition.
Method of administration
This medicine will be administered to the child through a small tube inserted into a vein (intravenous infusion).
When used in children from premature to 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration (see section 2).
Using a higher dose of Aminoplasmal Paed 10% than recommended
This is unlikely, as the doctor determines the daily doses for the child.
However, if the child receives a higher dose of the solution than recommended or the solution is administered too quickly, it may cause nausea, vomiting, and chills or headaches.
It may also lead to an increase in the level of acidic substances (metabolic acidosis) or ammonia (hyperammonemia) in the blood and to the loss of amino acids in the urine.
It may also cause an excess of fluid in the body (overhydration), disorders of mineral balance (electrolyte imbalance), and fluid may accumulate in the lungs (pulmonary edema). In such a case, the infusion will be discontinued and resumed later at a slower rate.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Such side effects are not clearly related to Aminoplasmal Paed 10%, but rather to parenteral nutrition in general, especially in the initial stage of treatment.
The following side effects may be serious. If the child experiences any of the following side effects, tell the doctor immediately, who will discontinue the administration of this medicine to the child:
Unknown (frequency cannot be estimated from the available data)
- allergic reactions
Other side effects
Uncommon (may occur in up to 1 in 100 patients)
- vomiting, nausea
Reporting side effects
If the child experiences any side effects, including any not listed in this leaflet, tell the doctor or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Aminoplasmal Paed 10%
The medicine should be stored out of sight and reach of children.
When used in children from premature to 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration (see section 2).
Do not use this medicine after the expiry date stated on the bottle or label.
The expiry date refers to the last day of the stated month.
There are no special precautions for storage.
Do not freeze.
After infusion, do not store the remaining solution for later use.
6. Contents of the packaging and other information
What Aminoplasmal Paed 10% contains
The active substances of the medicine are amino acids.
This medicine contains:
| Amino acids | in 1 ml | in 100 ml | in 250 ml |
| Isoleucine | 5.10 mg | 0.51 g | 1.28 g |
| Leucine | 7.60 mg | 0.76 g | 1.90 g |
| Lysine monohydrate (equivalent to lysine) | 9.88 mg (8.80 mg) | 0.99 g (0.88 g) | 2.47 g (2.20 g) |
| Methionine | 2.00 mg | 0.20 g | 0.50 g |
| Phenylalanine | 3.10 mg | 0.31 g | 0.78 g |
| Threonine | 5.10 mg | 0.51 g | 1.28 g |
| Tryptophan | 4.00 mg | 0.40 g | 1.00 g |
| Valine | 6.10 mg | 0.61 g | 1.53 g |
| Arginine | 9.10 mg | 0.91 g | 2.28 g |
| Histidine | 4.60 mg | 0.46 g | 1.15 g |
| Alanine | 15.90 mg | 1.59 g | 3.98 g |
| Glycine | 2.00 mg | 0.20 g | 0.50 g |
| Aspartic acid | 6.60 mg | 0.66 g | 1.65 g |
| Glutamic acid | 9.30 mg | 0.93 g | 2.33 g |
| Proline | 6.10 mg | 0.61 g | 1.53 g |
| Serine | 2.00 mg | 0.20 g | 0.50 g |
| N-acetyltyrosine (equivalent to tyrosine) | 1.30 mg (1.06 mg) | 0.13 g (0.11 g) | 0.33 g (0.27 g) |
| N-acetylcysteine (equivalent to cysteine) | 0.700 mg (0.520 mg) | 0.070 g (0.052 g) | 0.175 g (0.13 g) |
| Taurine | 0.300 mg | 0.030 g | 0.075 g |
| in 1 ml | in 100 ml | in 250 ml | |
| Total amino acid content | 0.1 g | 10 g | 25 g |
| Total nitrogen content | 0.0152 g | 1.52 g | 3.8 g |
Other ingredients are citric acid monohydrate (for pH adjustment) and water for injections.
What Aminoplasmal Paed 10% looks like and what the packaging contains
Aminoplasmal Paed 10% is a clear, colorless or slightly yellowish solution.
It is supplied in flexible bags with a capacity of 100 ml or 250 ml. The bags are made of multi-layered foil. The inner layer, in contact with the solution, is made of polypropylene.
The container does not contain polyvinyl chloride, DEHP, or latex.
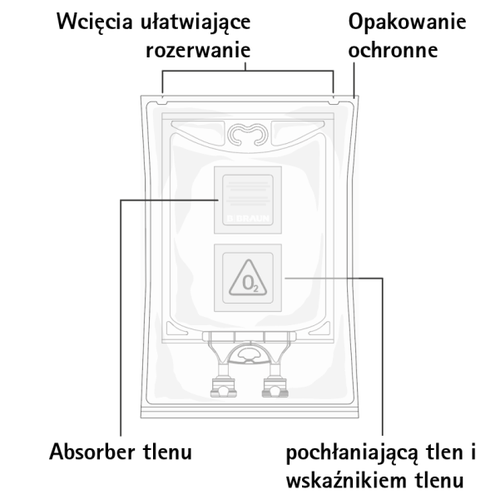
The bag is placed in a protective outer packaging. An oxygen absorber and an oxygen indicator are placed in the space between the bag and the protective packaging; the oxygen indicator is a thermoformed blister containing the oxygen-sensitive dye resorufin sodium;
the oxygen absorber sachet is made of a neutral material and contains iron hydroxide.
Boxes of 12 bags contain different sizes of containers. Packaging sizes: 12 x 100 ml and 12 x 250 ml.
Not all packaging sizes may be marketed.
Marketing authorization holder and manufacturer
- B. Braun Melsungen AG Carl-Braun-Straße 1 34212 Melsungen, Germany
Mailing address
34209 Melsungen, Germany
Phone: +49-5661-71-0
Fax: +49-5661-71-4567
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Energy value [kJ/l (kcal/l)] | 1700 (406) |
| Theoretical osmolality [mOsm/l] | 790 |
| Acidity (titration to pH 7.4) [mmol NaOH/l] | 23 |
| pH | about 6.1 |
Austria
Aminoplasmal Paed 10% Infusionslösung
Czech Republic
Amiped
Denmark
Amiped
France
Amiped, solution pour perfusion
Germany
Aminoplasmal Paed 10% Infusionslösung
Greece
Aminoplasmal Paed 10%
Italy
Amiped
Luxembourg
Aminoplasmal Paed 10%
Netherlands
Aminoplasmal Paed 100 mg/ml, oplossing voor infusie
Norway
Amiped
Poland
Aminoplasmal Paed 10%
Portugal
Aminoplasmal Paed, 100 mg/ml, Solução para perfusão
Slovakia
Amiped 10 % infúzny roztok
Slovenia
Aminoplasmal Paed 100 mg/ml raztopina za infundiranje
Spain
Aminoplasmal paed 10%, solucion para perfusion
United Kingdom
Aminoplasmal Paediatric 10% solution for infusion
Date of last revision of the leaflet: 2023-04-14
---------------------------------------------------------------------------------------------------------------------------
Information intended exclusively for healthcare professionals:
Dosage
Method of administration
Intravenous administration.
Only for central venous infusion.
When used in children from premature to 2 years of age, the solution (in the bag and administration set) should be protected from light until the end of administration.
During preparation of mixtures, the use of protective packaging that protects against light may be impossible. However, exposure to light during preparation of mixtures should be minimized as much as possible.
Children and adolescents
The dosing principles for age groups are approximate guideline values.
Exact doses should be adjusted individually based on age, development stage, and underlying disease.
Infusion should be started at a rate below the target infusion rate, increasing to the target rate within the first hour.
Supply of amino acids in parenteral nutrition considered suitable for most children:
Daily dose for premature newborns:
1.5-4.0 g of amino acids/kg of body weight ≙ 15-40 ml/kg of body weight
Daily dose for full-term newborns (aged 0 to 27 days):
1.5-3.0 g/kg of body weight ≙ 15-30 ml/kg of body weight
Daily dose for infants and small children (aged 28 days to 23 months):
1.0-2.5 g/kg of body weight ≙ 10-25 ml/kg of body weight
Daily dose for older children (aged 2 to 11 years):
1.0-2.0 g/kg of body weight ≙ 10-20 ml/kg of body weight
Special warnings and precautions for use:
Exposure of parenteral nutrition solutions containing Aminoplasmal Paed 10% to environmental light, especially after adding trace elements and/or vitamins, may have an adverse effect on the clinical effect in newborns, due to the formation of peroxides and other degradation products. When used in children from premature to 2 years of age, Aminoplasmal Paed 10% should be protected from environmental light until the end of administration.
Special precautions for disposal and preparation of the medicinal product for use
Use only a sterile infusion set for the solution Aminoplasmal Paed 10%.
Before opening the protective packaging, check the color of the oxygen indicator (see Figure A). Do not use if the oxygen indicator has turned pink. Use only if the oxygen indicator is yellow.
If it is necessary to add other nutritional components to this medicinal product, such as carbohydrates, fats, vitamins, electrolytes, and trace elements, addition of admixtures should be performed under strictly aseptic conditions. Mix thoroughly after adding any admixtures. Aminoplasmal Paed 10% can only be mixed with other nutritional components for which compatibility has been demonstrated. Information on compatibility for various admixtures and the corresponding shelf-life of such admixtures can be obtained on request from the manufacturer.
When used in children from premature to 2 years of age, solutions for parenteral nutrition containing Aminoplasmal Paed 10% should be protected from light until the end of administration. Exposure of such solutions to environmental light, especially after adding trace elements and/or vitamins, leads to the formation of peroxides and other degradation products, which can be limited by protecting against light.
Special precautions for storage
Use only if the solution is clear, colorless or slightly yellowish, does not contain solid particles, and the bottle and its closure are not damaged.
The containers are intended for single use only. After use, discard the protective packaging, oxygen indicator, oxygen absorber sachet, container, and any unused contents.
During preparation of mixtures
During preparation of mixtures, the use of protective packaging that protects against light may be impossible. However, exposure to light during preparation of mixtures should be minimized as much as possible.
Shelf-life after addition of admixtures
From a microbiological point of view, mixtures should be administered immediately after preparation. If the mixture is not administered immediately, the responsibility for the conditions and storage time of the mixture before administration lies with the user and usually should not exceed 24 hours at 2°C-8°C, unless the dilution has been carried out under controlled and validated aseptic conditions.
To obtain detailed information on this medicinal product, consult the Summary of Product Characteristics.
Aminoplasmal Paed 10%: Preparation of the medicinal product for use
Figure A:Bag and protective packaging
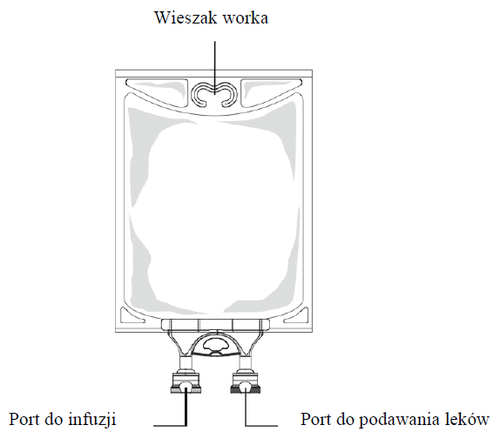
Figure B:Bag
To open:
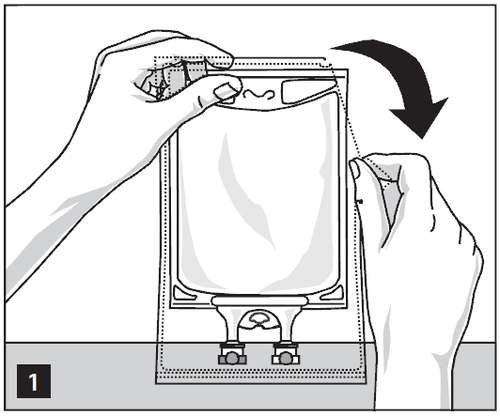
Remove the bag from the protective packaging by tearing the notches at the top and remove the container with the solution (Figure 1). Remove the protective packaging, oxygen absorber, and oxygen indicator. Check for leaks. If a leak is found, the product should be discarded as it may not be sterile.
To add medication:
Prepare admixtures following strict aseptic rules.
Compatible supplementary medicines can be added through the medication port (transparent).
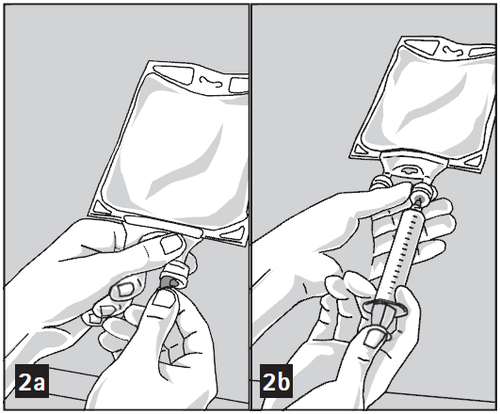
- 1. Prepare the medication port (transparent) by removing the aluminum foil (Figure 2a). Note: The area under the foil of the medication port is sterile.
- 2. Pierce the closable medication port and inject the additive(s) (Figure 2b).
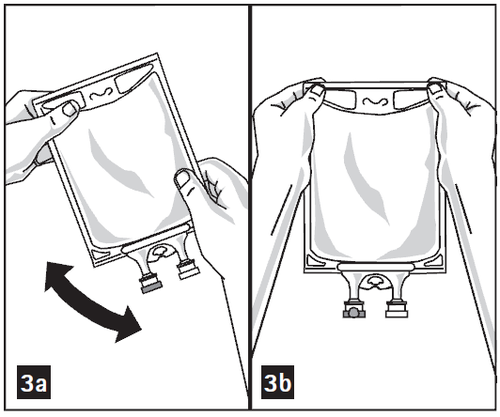
- 3. Mix the solution and medication thoroughly (Figure 3a).
- 4. Before piercing, the medication port can be wiped with a swab moistened with a disinfectant (e.g., isopropanol).
- 5. Visually inspect the admixture for solid particles (Figure 3b).
During preparation of mixtures
During preparation of mixtures, the use of protective packaging that protects against light may be impossible. However, exposure to light during preparation of mixtures should be minimized as much as possible.
Preparation for administration:
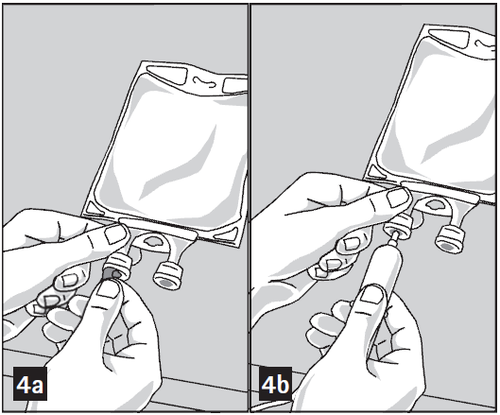
- 1. Remove the aluminum foil from the infusion port (green) at the bottom of the container (Figure 4a) and connect the administration set (Figure 4b): use an infusion set without a vent or close the ventilation opening of the set with a vent. Follow the instructions for use of the infusion set. Note: The area under the foil of the medication port is sterile.
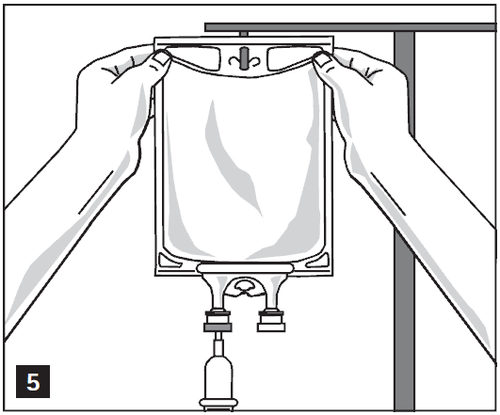
- 2. Hang the bag on an intravenous infusion stand (Figure 5).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterB. Braun Melsungen AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Aminoplasmal Paed 10%Dosage form: Solution, -Active substance: amino acidsManufacturer: Fresenius Kabi Deutschland GmbHPrescription not requiredActive substance: amino acidsManufacturer: B. Braun Melsungen AGPrescription requiredDosage form: Solution, -Active substance: amino acidsManufacturer: B. Braun Melsungen AGPrescription required
Alternatives to Aminoplasmal Paed 10% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aminoplasmal Paed 10% in Ukraine
Alternative to Aminoplasmal Paed 10% in Spain
Online doctors for Aminoplasmal Paed 10%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aminoplasmal Paed 10% – subject to medical assessment and local rules.














