
Ambroxol Rivopharm
Ask a doctor about a prescription for Ambroxol Rivopharm

How to use Ambroxol Rivopharm
Leaflet attached to the packaging: information for the user
Ambroxol Rivopharm, 30 mg/5 ml, oral solution
Ambroxol hydrochloride
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or additional information, consult a pharmacist.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
- If there is no improvement after 5 days or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Ambroxol Rivopharm and what is it used for
- 2. Important information before taking Ambroxol Rivopharm
- 3. How to take Ambroxol Rivopharm
- 4. Possible side effects
- 5. How to store Ambroxol Rivopharm
- 6. Contents of the packaging and other information
1. What is Ambroxol Rivopharm and what is it used for
Ambroxol Rivopharm contains the active substance ambroxol hydrochloride, which belongs to the group of mucolytics (anti-cough and anti-cold agents) that facilitate the clearance of airways.
Ambroxol Rivopharm, 30 mg/5 ml, is used to treat wet (productive) cough in adults and adolescents over 12 years old.
Wet cough is associated with lung and bronchial diseases characterized by excessive mucus production. Ambroxol Rivopharm works by thinning the mucus and reducing its viscosity, making it easier to cough up.
If there is no improvement after 5 days or the patient feels worse, they should consult their doctor.
2. Important information before taking Ambroxol Rivopharm
When not to take Ambroxol Rivopharm
- in children under 6 years of age.
Warnings and precautions
Before taking Ambroxol Rivopharm, consult a doctor or pharmacist:
Severe skin reactions associated with ambroxol administration have been reported. If the patient experiences a skin rash (including changes in the mucous membranes of the mouth, throat, nose, eyes, genitals), they should stop taking ambroxol and contact their doctor immediately.
Children
Ambroxol Rivopharm should not be taken by children under 6 years of age.
Ambroxol Rivopharm and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking, has recently taken, or might take.
No interactions with other medicines are known.
Taking Ambroxol Rivopharm with food
Ambroxol Rivopharm should be taken after meals, but can be taken during meals.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before taking this medicine.
Pregnancy
Ambroxol crosses the placenta to the fetus. Ambroxol Rivopharm should not be taken during pregnancy, especially during the first three months of pregnancy.
Breastfeeding
Ambroxol is excreted into human milk. Ambroxol Rivopharm should not be taken by breastfeeding women.
Driving and using machines
This medicine is unlikely to affect the ability to drive or use machines.
Ambroxol Rivopharm contains sorbitol
The medicine contains sorbitol (E420). If the patient has been told they have an intolerance to some sugars, they should consult their doctor before taking the medicine.
Each 5 ml dose of the oral solution contains 1.75 g of sorbitol.
3. How to take Ambroxol Rivopharm
This medicine should always be taken exactly as described in the patient leaflet or as directed by a doctor or pharmacist. If in doubt, consult a doctor or pharmacist.
Unless otherwise directed by a doctor, the recommended dose of Ambroxol Rivopharm is:
Adults and adolescents over 12 years old
For the first 2 to 3 days, take 5 ml of the oral solution three times a day (corresponding to 90 mg of ambroxol hydrochloride per day).
Then, take 5 ml of the oral solution twice a day (corresponding to 60 mg of ambroxol hydrochloride per day).
If necessary, in adults, the dose can be increased to 10 ml of the oral solution twice a day (corresponding to 120 mg of ambroxol hydrochloride per day).
Children between 6 and 12 years old
Other doses of this medicine may be more suitable for children between 6 and 12 years old; consult a doctor or pharmacist.
Children under 6 years old
Ambroxol Rivopharm should not be taken by children under 6 years old.
Patient with liver or kidney failure
Ambroxol Rivopharm should not be taken by patients with kidney failure or severe liver failure, unless directed by a doctor. The dose of the medicine or intervals between doses may need to be adjusted.
How to take
This medicine is for oral use only.
Ambroxol Rivopharm should be taken after meals, measuring the appropriate dose with the measuring cup (oral syringe).
It is recommended to drink a glass of water after taking the medicine and to drink plenty of fluids throughout the day.
If symptoms worsen or do not improve after 5 days, consult a doctor. Ambroxol Rivopharm should not be taken without medical advice for more than 4-5 days.
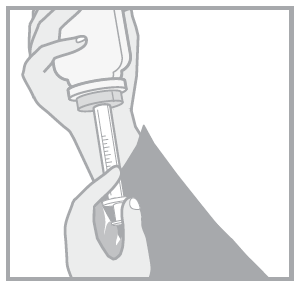
Follow the instructions to ensure proper dosing of the oral solution.
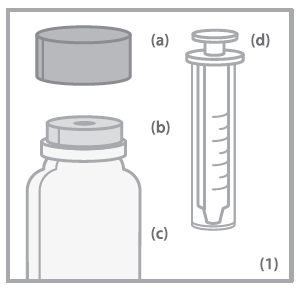
- (a) Child-resistant bottle cap
- (b) Bottle connector
- (c) Bottle
- (d) Oral syringe
- 1. Open the child-resistant cap by pressing and turning it in the opposite direction to the arrow.
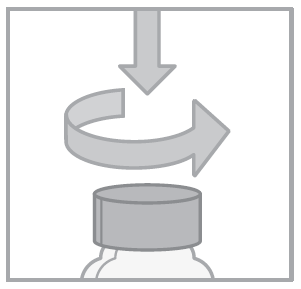
- 2. Press the syringe plunger.
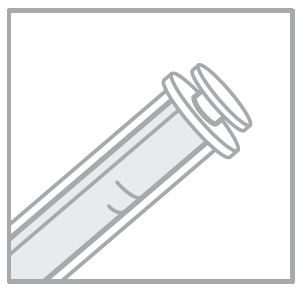
- 3. Insert the syringe firmly into the bottle connector.
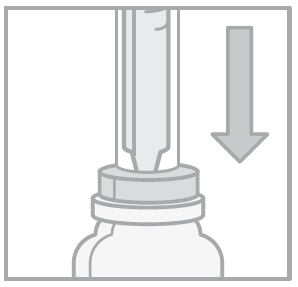
- 4. Turn the bottle and oral syringe upside down. Pull the syringe plunger until the correct dose is drawn.
Overdose of Ambroxol Rivopharm
No specific symptoms of overdose have been observed in humans to date. Based on cases of accidental overdose and/or reports of misuse, symptoms corresponding to the known adverse effects of ambroxol hydrochloride at recommended doses have been observed (see section 4). In case of accidental ingestion of a larger dose of the medicine, consult a doctor or go to the nearest hospital.
Take the leaflet and any remaining oral solution to the doctor or hospital to show which medicine was taken.
Missed dose of Ambroxol Rivopharm
If a dose is missed, take it as soon as possible, unless it is almost time for the next dose. If it is almost time for the next dose, skip the missed dose and take the next dose at the usual time.
Do not take a double dose to make up for a missed dose.
If you have any further questions on the use of this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following serious side effects occur, stop taking Ambroxol Rivopharm and contact a doctor immediately:
Frequency not known (frequency cannot be estimated from the available data):
- anaphylactic reactions, including anaphylactic shock, angioedema (rapidly spreading skin and mucous membrane swelling), and itching
- severe skin reactions (including erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, and acute generalized exanthematous pustulosis) (see Warnings and precautions in section 2).
Other side effects:
Common (may affect up to 1 in 10 people):
- nausea
- change in taste
- feeling of numbness in the mouth and throat (oral hypoesthesia).
Uncommon (may affect up to 1 in 100 people):
- vomiting
- dry mouth
- diarrhea
- indigestion
- abdominal pain.
Rare (may affect up to 1 in 1,000 people):
- hypersensitivity reactions
- rash, urticaria.
Frequency not known (frequency cannot be estimated from the available data):
- dry throat.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, inform a doctor or pharmacist. Side effects can be reported directly to the Department of Medicinal Product Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Jerozolimskie Avenue 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309,
e-mail: [email protected].
Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ambroxol Rivopharm
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and label after "EXP". The expiry date refers to the last day of the month.
The medicine does not require special storage conditions.
Shelf life after first opening: 6 months.
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Ambroxol Rivopharm contains
- The active substance is ambroxol hydrochloride. 1 ml of the oral solution contains 6 mg of ambroxol hydrochloride.
- The other ingredients are: sodium benzoate (E211), sorbitol (E420), sucralose, hydroxyethylcellulose, citric acid monohydrate (E330), purified water, and strawberry flavor (501440 T) (propylene glycol (E1520), flavoring substances).
What Ambroxol Rivopharm looks like and contents of the pack
Ambroxol Rivopharm, oral solution is a colorless or pale yellow liquid with a strawberry odor.
The bottle is made of orange glass (type III) with a child-resistant closure made of HDPE, with an outer cap made of PP and a connector made of PE. The pack includes an oral syringe made of PP (5 ml syringe with 0.5 ml graduations) with a plunger made of HDPE.
Pack size: 100 ml of the oral solution.
An oral syringe is included in the pack.
Not all pack sizes may be marketed.
Marketing authorization holder
Actavis Group PTC ehf.
Reykjavíkurvegi 76-78
220 Hafnarfjörður
Iceland
Manufacturer
Balkanpharma-Troyan AD
1, Krayrechna Str.
5600 Troyan
Bulgaria
For further information on the medicine and its names in the Member States of the European Economic Area, please contact the representative of the marketing authorization holder:
Actavis Export International Limited, BLB 016 Bulebel Industrial Estate, ZTN3000, Zejtun, Malta.
Contact in Poland: phone (+48 22) 512 29 00.
Date of last revision of the leaflet:January 2020
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterBalkanpharma-Troyan AD
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ambroxol RivopharmDosage form: Solution, 7.5 mg/mlActive substance: ambroxolManufacturer: Przedsiębiorstwo Farmaceutyczne Jelfa S.A.Prescription requiredDosage form: Syrup, 15 mg/5 mlActive substance: ambroxolManufacturer: Sopharma PLCPrescription not requiredDosage form: Syrup, 30 mg/5 mlActive substance: ambroxolPrescription not required
Alternatives to Ambroxol Rivopharm in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ambroxol Rivopharm in Hiszpania
Alternative to Ambroxol Rivopharm in Ukraina
Online doctors for Ambroxol Rivopharm
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ambroxol Rivopharm – subject to medical assessment and local rules.














